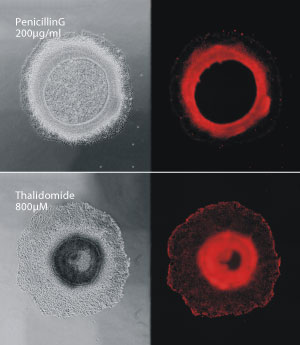
Compounds that cause birth defects, such as thalidomide (bottom), disrupt the ring-like formation of colonies of human stem cells, compared with compounds that are not toxic to the developing fetus, such as penicillin G (top).
Reproduced from Ref. 1 and licensed under CC BY 4.0 © 2015 J. Xing et al.
Researchers at A*STAR have designed a highly sensitive, animal-free method to test the toxicity of drugs on developing embryos. The technique can identify compounds that disrupt the differentiation and, for the first time, the movement of growing colonies of human stem cells.
“Our technique detects two essential processes of embryonic development: cellular differentiation and migration,” says Hanry Yu, who led the research together with his colleagues at the A*STAR Institute of Biotechnology and Nanotechnology. “This greatly enhances our ability to predict drug-induced toxicity during development.”
Drugs found to cause birth defects are categorized as teratogens. These include the sedative, thalidomide, initially prescribed to pregnant women to combat morning sickness until it was found to disrupt limb formation in growing fetuses.
Researchers have typically tested the teratogenic effects of various compounds on animals, which is expensive, time-consuming and contested on ethical grounds. The main alternative looks at how these compounds disrupt the differentiation of mouse embryonic stem cells into beating heart muscle cells, often with unreliable results.
Yu’s team instead developed a drug screening model using human pluripotent stem cells that differentiate into circular structures resembling the earliest cellular layers formed during embryogenesis. The model could be used to observe teratogenic changes over time, and more significantly, space.
In cell culture experiments, the researchers found that colonies treated with thalidomide tended to migrate closer to the center of the ring, whereas those treated with non teratogenic penicillin G migrated outward to form thin rings similar to those produced by untreated cells (see image).

Postdoctoral Fellow Jiangwa Xing (seated) and Group Leader Hanry Yu at the A*STAR Institute of Biotechnology and Nanotechnology.
© 2015 A*STAR Institute of Biotechnology and Nanotechnology
“Anyone studying embryonic biology will know that drugs that affect cellular migration in the embryo cause defects: the heart will be in the wrong place or the brain will be in the wrong place or no such organs will grow at all,” says Yu. “But nobody was paying attention to this migratory pattern in drug testing.”
The researchers compared the performance of their cells against mouse cardiomyocytes in classifying four known teratogens and one non teratogen. The mouse model misclassified two of the five drugs, including thalidomide, which only affects human and not mouse development. Yu’s model, however, identified all five drugs correctly.
The researchers have since tested their model on 11 other drugs, with close to 100 per cent predictability of teratogenicity, and plan to test another 30 with funding from A*STAR’s technology transfer arm, ETPL (Exploit Technologies Pte Ltd). “Once we reach 50 or more compounds, the community will be convinced,” says Yu, describing a pharmaceutical industry eager to cut its losses on drugs found to be harmful to health.
The A*STAR-affiliated researchers contributing to this research are from the Institute of Biotechnology and Nanotechnology.




