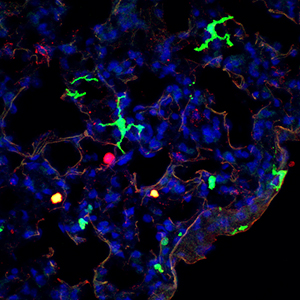
Discovery of the CD11b+ subset (green) of murine dendritic cells and its direct human counterpart (not shown) could lead to improved therapeutics.
© 2013 A*STAR Singapore Immunology Network
For the human immune system to work effectively, the body must be able to distinguish invading pathogens, such as fungi and bacteria, from its own healthy tissue. A group of white blood cells known as dendritic cells (DCs) has a critical role in this task: DCs recognize pathogens, then activate and regulate the immune system accordingly. Immunologists therefore believe that DCs could be harnessed for new therapies against fungal and bacterial infections, as well as autoimmune diseases such as multiple sclerosis.
Florent Ginhoux and co-workers at the A*STAR Singapore Immunology Network, together with researchers in the United Kingdom, United States and Japan, have identified a subset of DCs that exists in mouse and human mucosal tissues. The murine subset is called CD11b+ and the human equivalent is called CD1c+. The existence of similar cell populations in the two species will assist in translating the results of murine experiments to human biology and also further the development of clinical therapies.
Contamination by other cell types in previous studies limited the true understanding of the function of CD11b+ cells, Ginhoux notes. “In this study, we found new markers to specifically identify CD11b+ DCs.”
Parts of the body that are exposed to the external environment, such as the lungs and gut, contain DCs (see image). They act as messengers, presenting fragments of pathogens to other white blood cells called CD4 T-helper cells that trigger appropriate immune responses. One particular T-helper, Th17, in concert with DCs, specializes in activating the protective response to fungal or bacterial infections.
“We found that CD11b+ DCs secrete a specific cytokine protein named interleukin-23 (IL-23). This protein induces and governs Th17 cells that secrete a second cytokine — IL-17 — a very potent inflammatory mediator against fungi in the lungs of mice as well as of humans,” explains Ginhoux.
During infections that fight and clear pathogens, IL-17 can be very powerful. If cytokine IL-23 secretion is unregulated, however, it induces exacerbated Th17 responses because of an excess of IL-17 release. This regulation failure has been linked to the development of psoriasis, Crohn’s disease and multiple sclerosis. Controlling the activity of DCs that regulate IL-23 and subsequent Th17 cell responses could therefore prove useful therapeutically.
“There are two major applications for this research,” says Ginhoux. Firstly, a vaccine strategy targeting CD11b+ DCs to trigger a potent IL-17-dependent immune response could prevent fungal and bacterial infections. Secondly, selectively inhibiting CD11b+ DCs may lead to better control of IL-17-dependent autoinflammatory disorders.
The A*STAR-affiliated researchers contributing to this research are from the Singapore Immunology Network and the Singapore Institute for Clinical Sciences.



