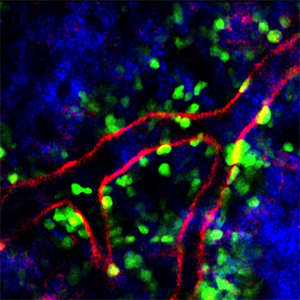
Plerixafor increases the number of neutrophils in the blood, in part, by preventing them from being trafficked back into the bone marrow environment, shown here with neutrophils in green, blood vessels in red and collagen fibers in blue.
© 2013 A*STAR Singapore Immunology Network
People with low numbers of neutrophils, a type of white blood cell, are prone to chronic or even life-threatening infections. Many patients with the condition ‘neutropenia’ are treated with a drug known as granulocyte colony-stimulating factor (G-CSF). This drug stimulates the bone marrow to release more neutrophils into the bloodstream but remains an imperfect treatment. Recently, researchers have found that another agent called plerixafor can increase neutrophil levels in people with a rare congenital immunodeficiency disorder called WHIM (warts, hypogammaglobulinemia, infections and myelokathexis) syndrome. However, just how plerixafor promotes neutrophil mobilization remained unclear until now.
A team led by A*STAR researchers has teased apart the mechanism by which plerixafor exerts its therapeutic benefit. Using a combination of mouse models coupled with advanced imaging techniques, the researchers demonstrated that, unlike G-CSF, plerixafor facilitates neutrophil release from the lungs at the same time that the drug blocks neutrophil trafficking back into the bone marrow.
“Our data provide the first direct evidence that G-CSF and plerixafor induce blood neutrophilia by mobilizing these cells from distinct reservoirs via contrasting mechanisms,” says Lai Guan Ng, from the A*STAR Singapore Immunology Network, who led the work.
Ng and his co-workers used a cutting-edge technology known as intravital multiphoton imaging to delineate the effects of G-CSF and plerixafor on neutrophils in various body compartments. They observed that while blood neutrophil frequency increased after treatment with either G-CSF or plerixafor, only G-CSF increased neutrophil motility from the bone marrow.
Meanwhile, plerixafor enlarged the pool of circulating neutrophils through two distinct mechanisms: the drug promoted neutrophil release from a reservoir present in lung tissue and simultaneously blocked neutrophils from circulating back to the bone marrow (see image). The research provides the first evidence that major changes in blood neutrophils can originate from cell populations outside the bone marrow.
According to Ng, the findings could underpin the design of better treatment regimens for neutropenia. “A combined treatment of G-CSF and plerixafor could be beneficial,” Ng says. “G-CSF treatment can augment circulating neutrophils by increasing neutrophil release to the blood from the bone marrow, and plerixafor treatment can increase the retention of these additional neutrophils in the blood compartment.”
Currently, G-CSF and plerixafor are only used together to mobilize hematopoietic stem cells (HSCs) in cancer patients preparing to undergo transplantation therapy. “In light of our current findings,” Ng notes, “it will be interesting to determine whether plerixafor-induced HSC mobilization derives solely from the bone marrow.”
The A*STAR-affiliated researchers contributing to this research are from the Singapore Immunology Network.




