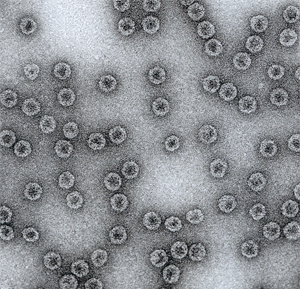
A scanning electron micrograph of Cytos’ virus-like particle (VLP).
© 2013 Cytos Biotechnology AG
Continuing population growth and urbanization are likely to increase the transmission of infections in Singapore. Higher levels of migration, travel and tourism are also intensifying the nation’s susceptibility to disease transmission. Drawing on experiences from the influenza, or ‘flu’, outbreaks of recent years, many current research efforts have turned their focus to the development of influenza vaccines.
In late May 2009, following a global outbreak of the H1N1 influenza A virus (H1N1), the first confirmed case in Singapore was diagnosed. Despite the rigorous containment measures enforced by the government, the number of confirmed cases rose to 1,217 within 6 weeks. By June that year, the World Health Organization (WHO) declared the new strain — widely referred to as ‘swine flu’ — a pandemic. Three months later, when the Singapore Ministry of Health (MOH) had managed to secure a supply of vaccine from healthcare company GlaxoSmithKline, the pandemic was already in decline. Left with an excess of a vaccine that had a short expiry date, the following year the MOH decided to make their supply available to the public at no cost.
While the H1N1 pandemic was relatively mild, it did have a notable impact on the tourism and retail sectors of Singapore. If the outbreak had been more severe, like that of severe acute respiratory syndrome (SARS) in 2003, the economic and public health consequences could have been dire. These two events served to highlight the vulnerability of Singapore to infectious diseases and the nation’s dependence on overseas vaccine manufacturers. Thus, to better prepare for potential future pandemics, it has become vital for Singapore to be able to quickly and locally produce large quantities of vaccine.
Singapore’s very own vaccine
The A*STAR Experimental Therapeutics Centre (ETC) and Cytos Biotechnology AG, a Swiss biopharmaceutical company with a focus on vaccine development, have forged a partnership to create a vaccine that offers protection against H1N1. “This is the first time Singapore is attempting to make its own flu vaccine,” says Lim Chuan Poh, chairman of A*STAR, who believes the attempt will improve Singapore’s ability to respond to flu outbreaks. The choice of H1N1 as the vaccine’s target strain was based on a recommendation by the WHO.
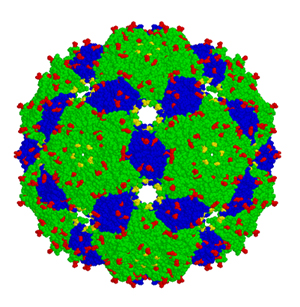
Schematic representation of the Qβ VLP (blue and green) before coupling to hemagglutinin, one of the proteins present on the surface of influenza viruses that elicit an immune response in humans.
© 2013 Cytos Biotechnology AG
Influenza, a contagious respiratory illness caused by influenza viruses, spreads easily from person to person and can affect people of any age, leading to mild to severe illness or, at times, even death. Two major proteins on the surface of influenza viruses — neuraminidase (N) and hemagglutinin (H) — are responsible for eliciting a protective antibody response in humans. Flu viruses are characterized by the types of neuraminidase and hemagglutinin that they carry, which give rise to the names of influenza subtypes, such as H1N1 and H7N9. Conventionally, chicken eggs are used to manufacture flu vaccines, but this process is time-consuming, presents limited yields and is sometimes constrained by the toxicity of certain viral strains to birds.
The new H1N1 vaccine is based on Cytos’ bacteriophage Qβ virus-like particle (VLP) technology. VLPs are multi-protein structures that resemble viruses but are noninfectious due to the absence of viral genetic material. The VLP platform was chosen as the basis for the vaccine as it offers several advantages, including the easy production of influenza-subtype hemagglutinins and VLPs by recombinant techniques, which eliminates the need to work with live viruses. Furthermore, the entire process is relatively fast and economical.
Clinical trial begins
In April 2013, the first healthy volunteer was dosed with the vaccine candidate in a Phase 1 clinical trial, which will evaluate the safety and immunogenicity of the vaccine. “In the wake of the recent H7N9 bird flu outbreak, it is timely that A*STAR is taking Singapore’s first H1N1 flu vaccine into clinical trial,” says Lim. Christian Itin, chairman and CEO of Cytos, agrees: “We are very pleased with the fruitful collaboration, which has led to the clinical start of this novel influenza vaccine. This is an important milestone for the program and the first clinical program using Cytos’ B-cell vaccine platform for a prophylactic vaccine against infectious disease.”
In addition to the ETC and Cytos, several other local academic and clinical partners are participating in the research effort. The immunological aspects were tackled by researchers at the A*STAR Singapore Immunology Network (SIgN) while the ETC acted as the primary driver at the start of the clinical development program. Nonclinical efficacy experiments were performed at the DSO National Laboratories and Duke–NUS Graduate Medical School. In early 2012, ‘D3’, the A*STAR drug discovery and development platform, collaborated with Cytos to lead the development of the vaccine project with the aim of achieving ‘proof-of-concept’ in humans. At present, a clinical trial is being conducted at the SingHealth Investigational Medicine Unit and the Changi General Hospital Clinical Trials & Research Unit.
Overall, the team aims to include 80 healthy individuals in their first clinical trial. “If this VLP-vaccine strategy proves to be effective, it can accelerate the production of vaccines against new, emerging strains of flu,” says Alex Matter, professor and CEO of D3 and the ETC. “In the event of a flu epidemic, we hope to contribute to Singapore’s preparedness by producing vaccine candidates for clinical trials quickly, safely and economically.”
Opening new doors for vaccine production
While it is still early days for the project, the team takes pride in the progress made so far. Their efforts demonstrate that a cohesive team can be successfully formed from different institutions to work toward a common goal. In addition to overcoming great technical and clinical challenges, the team has overcome financial hurdles and secured funding for the project through grants from ETPL (Exploit Technologies Pte Ltd), the technology transfer arm of A*STAR, and SIgN, which together with the ETC also made substantial in-kind, as well as financial, contributions. “This experience, in our view, augurs well for the expertise, the will and the stamina of Singapore R&D to tackle ambitious projects in the biomedical arena,” shares Matter.
A successful outcome for the trial will no doubt open up new opportunities to tackle emerging flu strains. “I am pleased that the collaboration with Cytos is making a meaningful contribution to Singapore’s pandemic readiness, a critical aspect of our national security,” says Lim. If the vaccine is approved for mass production, A*STAR subsidiaries will have the right to develop and commercialize the vaccine for Singapore and other southeast Asian countries and can earn royalties on worldwide net sales. “The potential success of this vaccine will have a significant impact not only in the region but globally as well,” adds Lim.
About the Singapore Immunology Network
The Singapore Immunology Network (SIgN) was launched by A*STAR with the aim of expanding and strengthening immunology research in Singapore by advancing human immunology research and participating in international efforts to combat major health problems. Researchers at SIgN investigate immunity during infections and inflammatory conditions, including cancer, using mouse models and human tissue and integrate the findings into clinical applications. Since its launch, SIgN has grown rapidly, and currently includes around 250 scientists from 26 countries working under 28 renowned principal investigators and supported by cutting-edge technological research platforms and core services.
About the Experimental Therapeutics Centre
The A*STAR Experimental Therapeutics Centre (ETC) was established in 2007 as a center of excellence to advance drug discovery and development in Singapore. The ETC addresses unmet medical needs, primarily in the fields of oncology and infectious diseases, through collaborative public-private partnerships in order to translate early-stage discoveries into clinical applications. The Centre also organizes educational programs and provides mentoring and support for staff, researchers, clinicians and students interested in drug development.
About the D3 platform
The A*STAR D3 platform was established in 2012 to build strong bridges between basic science and clinical research and development by bringing early-stage scientific discoveries to ‘proof-of-concept’ clinical trials in humans and generating economic benefit through the licensing of clinical stage therapeutics. D3 builds on Singapore’s existing drug discovery capabilities and strengthens the local biomedical innovation landscape. It was founded to be a cost-effective and professional development partner able to advance and add value to early-stage projects on a ‘shared-risk, shared-reward’ basis. D3’s primary focus is on developing drugs targeted at oncology indications and infectious diseases but the door is open to other indications if a partner brings the necessary disease knowledge. D3 is jointly funded by A*STAR, the Singapore Ministry of Health’s National Medical Research Council and the National Research Foundation.
About Cytos Biotechnology AG
Cytos Biotechnology AG is a public biopharmaceutical company focused on the development of targeted immunotherapies. The company’s lead product candidate, CYT003, is a novel, first-in-class, immune modulator in Phase 2 clinical development as a potential new treatment for asthma. CYT003 has a novel mechanism of action that inhibits the immune response that causes asthma and may therefore be beneficial for the control of asthma. In a successfully completed Phase 2a study, CYT003 was shown to maintain asthma control and lung function in patients with persistent allergic asthma despite withdrawal of standard therapy with inhaled corticosteroids. CYT003 has been shown to have a good safety and tolerability profile in more than 450 individuals receiving the active agent so far. Cytos was founded in 1995 as a spinoff from the Swiss Federal Institute of Technology (ETH Zurich) and is listed according to the Main Standard on the SIX Swiss Exchange Ltd under the symbol CYTN.



