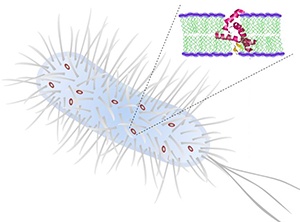
Synthetic α-helical peptides kill bacteria by penetrating and disrupting the cell membrane
© 2011 IBN
Antimicrobial peptides (AMPs) produced by the innate immune system provide an effective first line of defense against recurring microbial infections. Their structures are used as blueprints in the development of new drugs, but the drugs thus produced can compromise immunity if their amino sequences resemble those of the host organism’s AMPs too closely.
Yiyan Yang at the A*STAR Institute of Bioengineering and Nanotechnology and co-workers have now used the principles of material design to synthesize a series of synthetic α-helical AMPs that could overcome these problems as well as providing insights into the antimicrobial activity of AMPs.
The synthetic peptides created by Yang and her co-workers have a general structure consisting of two amino acid residues that are repelled by water bonded to two positively charged residues (see image), with this basic unit repeated to generate peptides of various lengths. The peptides mimic the folding behavior of naturally occurring AMPs, with a regular turn every 3.6 amino acid residues to form a stable α-helical structure held together by hydrogen bonding.
As standard ‘hemolytic’ test for evaluating the toxicity of antimicrobial drugs, Yang and her co-workers determined the concentration of each peptide needed to burst open 50% of red blood cells in a culture dish. They found that peptides with three repeat units destroyed fewer than 50% of red blood cells at concentrations of up to 500 milligrams per liter, whereas those with four repeat units damaged more than 60% of cells at concentrations more than ten times lower. The researchers consider that the increased hemolytic effect of the longer peptides is probably due to the increased backbone rigidity of the longer α-helical peptide, leading to a greater ability to penetrate and disrupt the membranes of the red blood cells.
The effect of the peptides was then tested against the Gram-positive bacterium Baccilus subtilis and the yeast Candida albicans, both of which are common causes of infection. Most of the peptides effectively suppressed both microbes, but those with three repeat units did so at concentrations that destroyed few red blood cells.
One of the synthetic peptides, consisting of two lycine and two leucine residues repeated three times, effectively killed both microbes at a concentration that destroyed less than 5% of red blood cells, and did not induce a non-specific immune response, suggesting that it could be a promising new antimicrobial agent. “We have further optimized the peptide design to improve on antimicrobial spectrum and activity,” says Yang. “This has resulted in peptides that can also kill Gram-negative pathogenic bacteria such as Escherichia coli and Pseudomonas aeroginosa.”
The A*STAR-affiliated researchers contributing to this research are from the Institute of Bioengineering and Nanotechnology.



