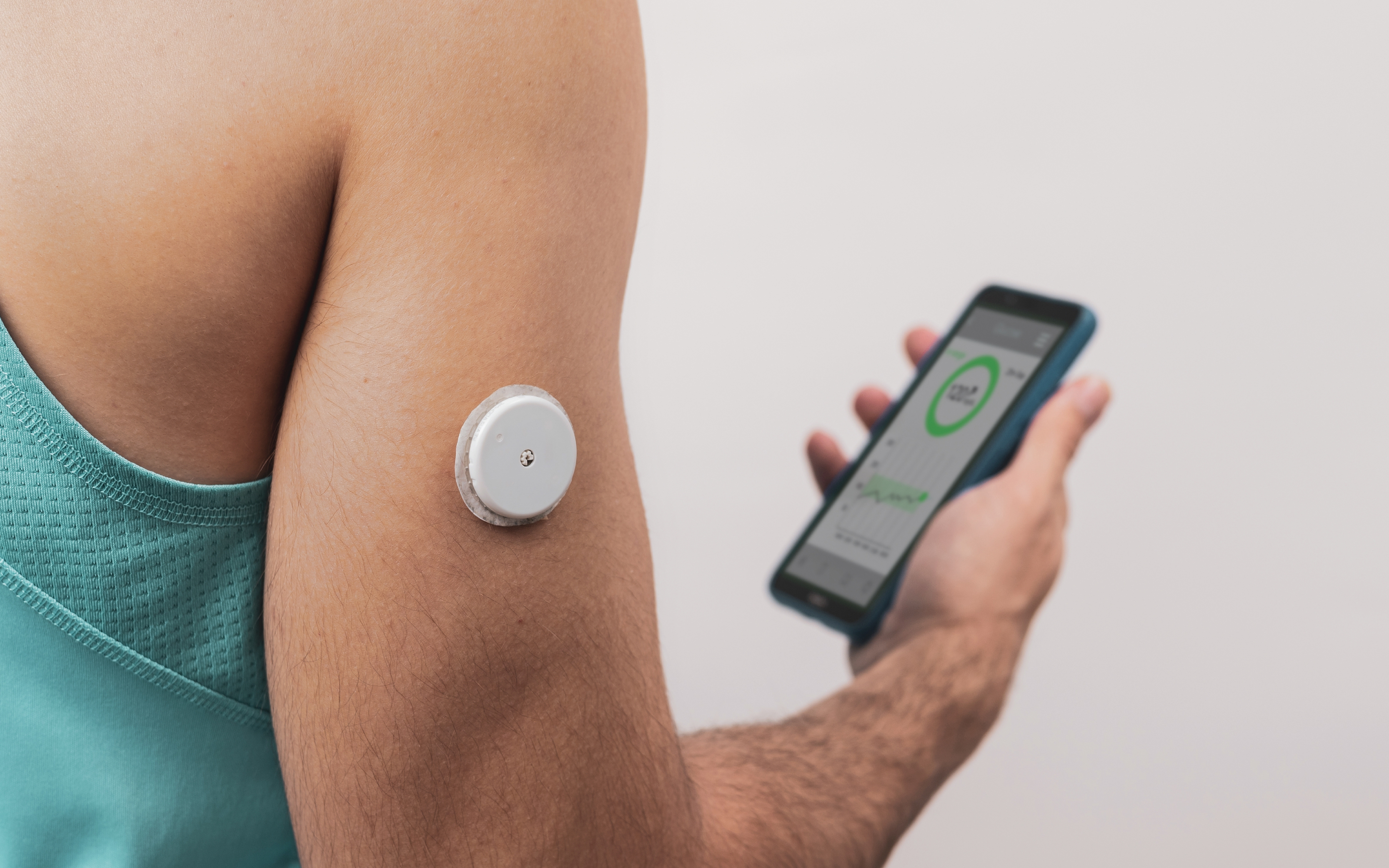
Whether as sleek smartwatches or blocky wristbands, fitness trackers are an increasingly common sight in Singapore, as even public initiatives like the National Steps Challenge promote their adoption. These devices could soon evolve beyond rigid cases into soft, skin-conforming patches, allowing users to wear them comfortably day and night.
However, flexible bioelectronics like these could also create new environmental issues, according to Zibiao Li, Director of the Resource Circularity Division at the A*STAR Institute of Sustainability for Chemicals, Energy and Environment (A*STAR ISCE2). Even today, simpler soft sensors—such as electrodes for heart monitors—mostly end up in landfill due to their non-recyclable plastic content.
“The growing environmental concerns associated with electronic waste highlight a pressing need for sustainable alternatives,” said Li.
Li added that soft polymers such as ionogels are currently a promising base material for flexible bioelectronics. Unlike traditional counterparts such as hydrogels, ionogels are more durable and electrochemically stable, making them ideal for long-term outdoor use. Some are even recyclable—but at a cost.
“Existing recyclable ionogels are held together by reversible, non-covalent bonds, which typically reduce their strength and stability,” said Xian Jun Loh, Executive Director at the A*STAR Institute of Materials Research and Engineering (A*STAR IMRE). “This significantly limits their practical use in wearables that demand durability and flexibility.”
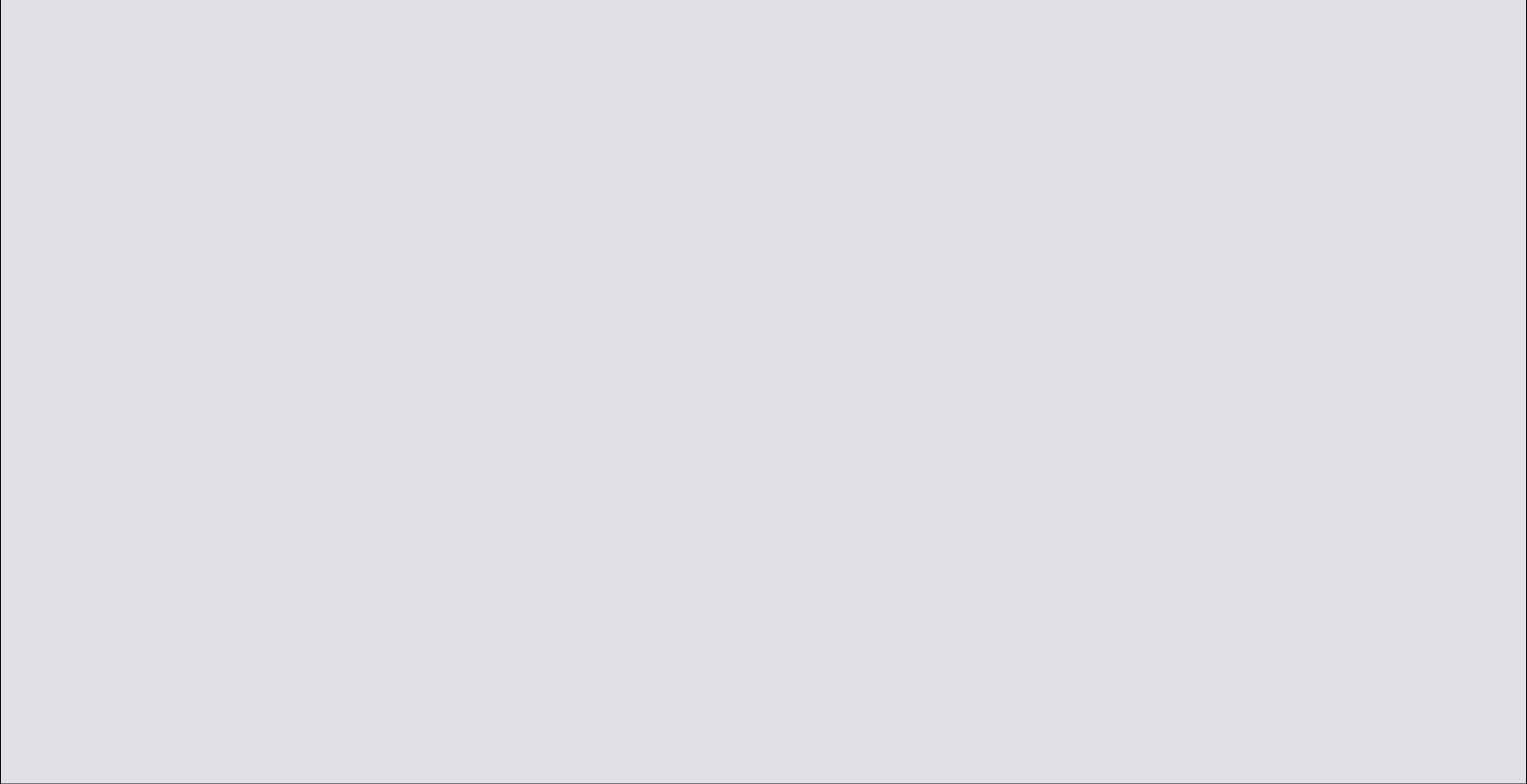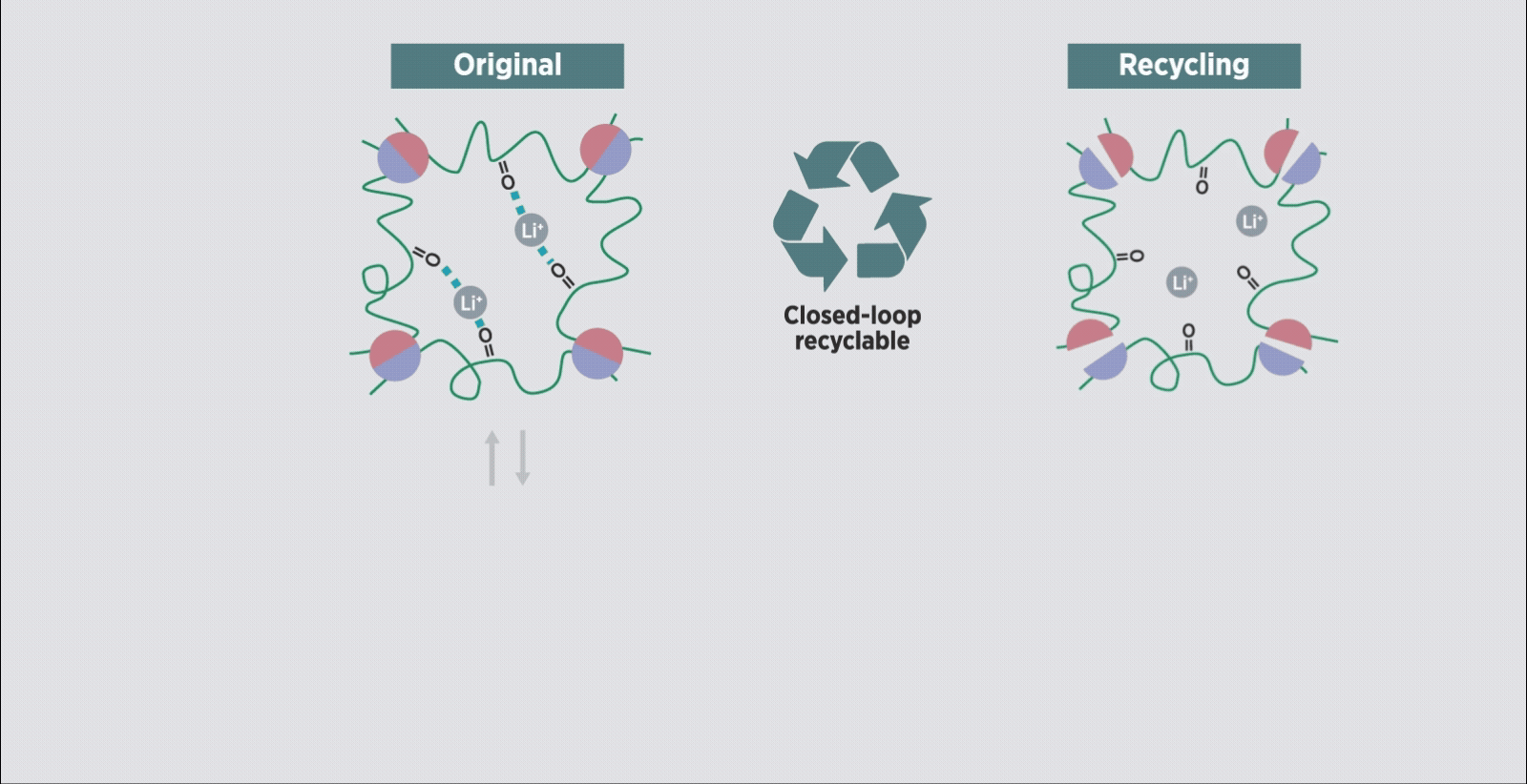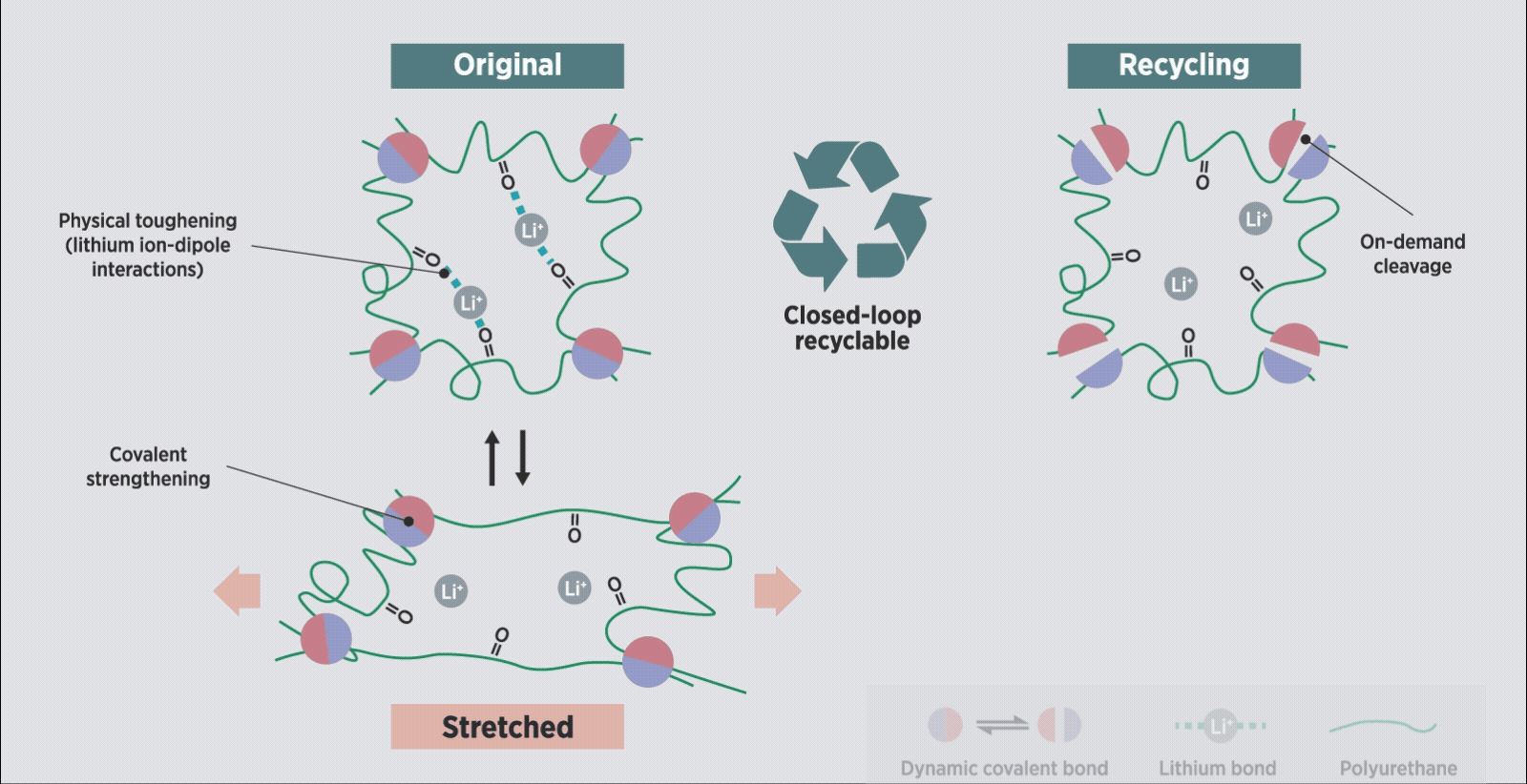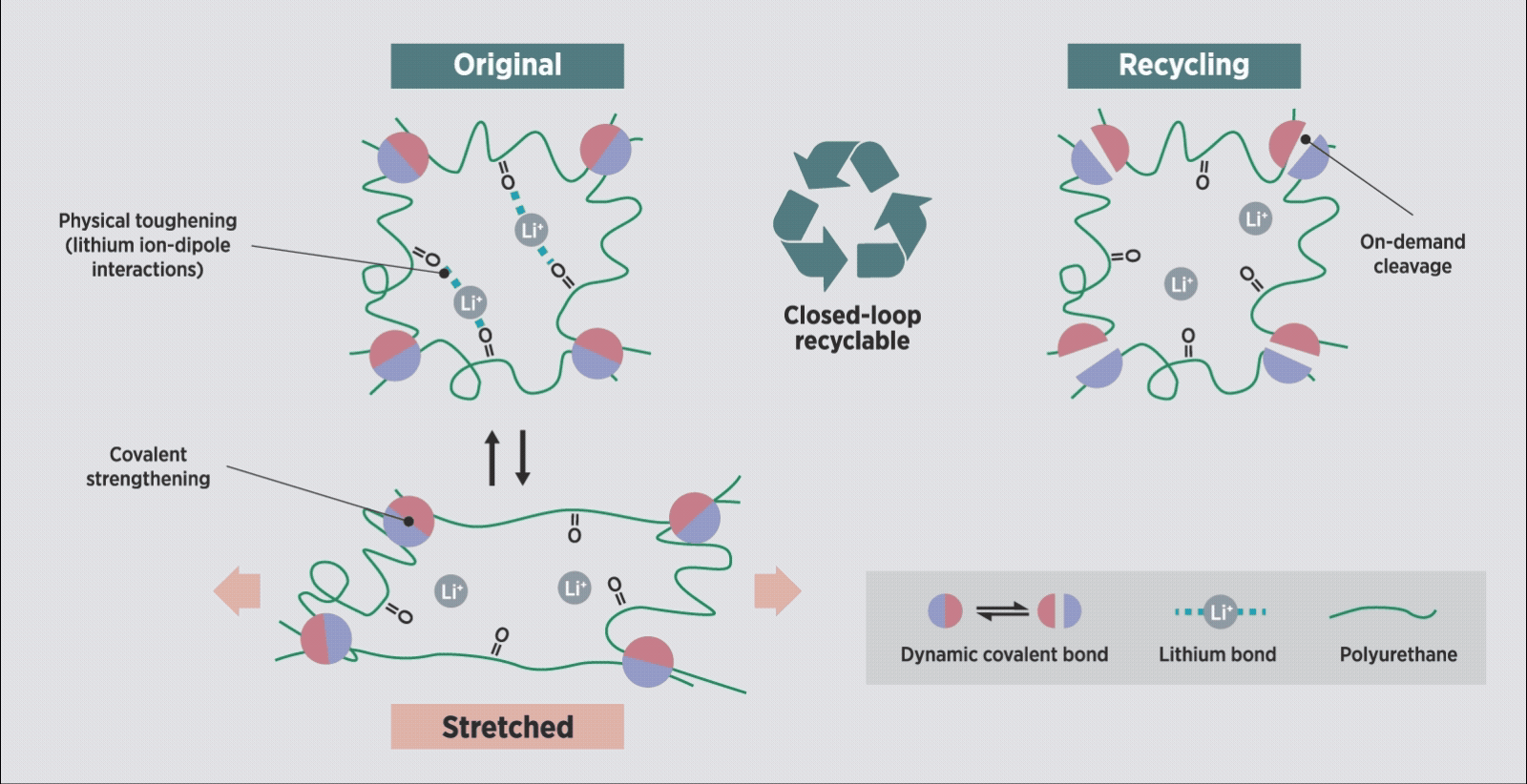
Schematic illustration of the proposed polymer network design for a robust and recyclable ionogel, based on covalent adaptable networks.
© A*STAR Research
In collaboration with Nanyang Technological University, Singapore, Li, Loh and colleagues including A*STAR ISCE2 Scientist Xiaotong Fan and A*STAR IMRE Senior Scientist Yifei Luo aimed to tackle that performance-recyclability tradeoff. Combining expertise in materials sustainability and polymer chemistry, the
team explored a new ionogel system based on covalent adaptable networks (CANs).
CANs contain dynamic bonds which allow their mesh-like polymer structure to temporarily break down at certain heat, light or pH levels, then reform once those stimuli are removed, all without the need for toxic catalysts. “This strategy allowed us to design a closed-loop, recyclable ionogel system that also exhibits robust
mechanical properties,” said Fan, the study’s lead author.
The result was a transparent film that proved not only physically stronger than other reported recyclable ionogels, but on par with non-recyclable designs. The team found that a 0.05 g film, measuring 0.2 mm thick and 0.3 cm wide, could easily lift a 1 kg object. The ionogels also maintained their properties after up to 10 rounds of recycling.
The team was surprised to find that lithium ions, originally added to improve their system’s conductivity, also boosted its mechanical strength by ‘gelling’ it together. Ion-dipole interactions made the lithium ions act both as a bonding agent and a conductor, creating a ‘sweet spot’ of performance and durability.
“These interactions resolve another long-standing tradeoff in ionogels; one between their electrical and mechanical properties,” said Luo.
Moving forward, the team aims to upgrade the adhesive properties of recyclable ionogels. “Stable long-term skin contact would enhance user comfort and ensure more accurate health monitoring, especially during motion and perspiration,” Luo added.
The A*STAR-affiliated researchers contributing to this research are from the A*STAR Institute of Sustainability for Chemicals, Energy and Environment (A*STAR ISCE2) and A*STAR Institute of Materials Research and Engineering (A*STAR IMRE).