Ever noticed how the longer you own a smartphone, the more frequently you need to charge it? This is because the lithium rechargeable batteries that power many of today’s technologies have fixed lifespans, with their capacity for storing electricity waning over time.
This phenomenon—known as cycle instability—along with issues of cost, hazards and the scarcity of raw materials for lithium batteries have spurred the search for alternative power sources. With their ready availability and safety, zinc-ion batteries (ZIBs) overcome many limitations of traditional lithium batteries. In ZIBs, positively charged zinc ions travel from the batteries’ zinc metal anode to the vanadium oxide-based cathode.
Unfortunately, zinc-ion batteries still suffer from cycle instability. Strong interactions between embedded O2– ions and the zinc charge carriers hinder the diffusion of zinc ions across the battery, gradually compromising recharging efficiency and weakening the battery capacity of ZIBs over time.
To unlock more durable, high-performing ZIBs, disrupting these molecular interactions could be key, explained Zhigen Yu, Senior Research Scientist at A*STAR’s Institute of High Performance Computing (IHPC). In a collaboration with Junmin Xue and Vincent Lee from the National University of Singapore’s Department of Materials Science and Engineering, Yu and a team of researchers explored novel ZIB design approaches to achieve superior battery performance.
They hypothesized that introducing trace amounts of carbon dioxide, CO2, to the vanadium oxide framework could reduce the zinc ion diffusion barrier. Previously, water molecules had been used for this purpose, but the team speculated that CO2 may provide ZIBs with an even greater functional boost.
“CO2 is a small and abundant non-polar molecule consisting of one carbon and two oxygen atoms,” said Yu, who explained that theoretically, the two oxygen atoms would ‘pull’ the zinc charge carriers along, ferrying them across the battery.
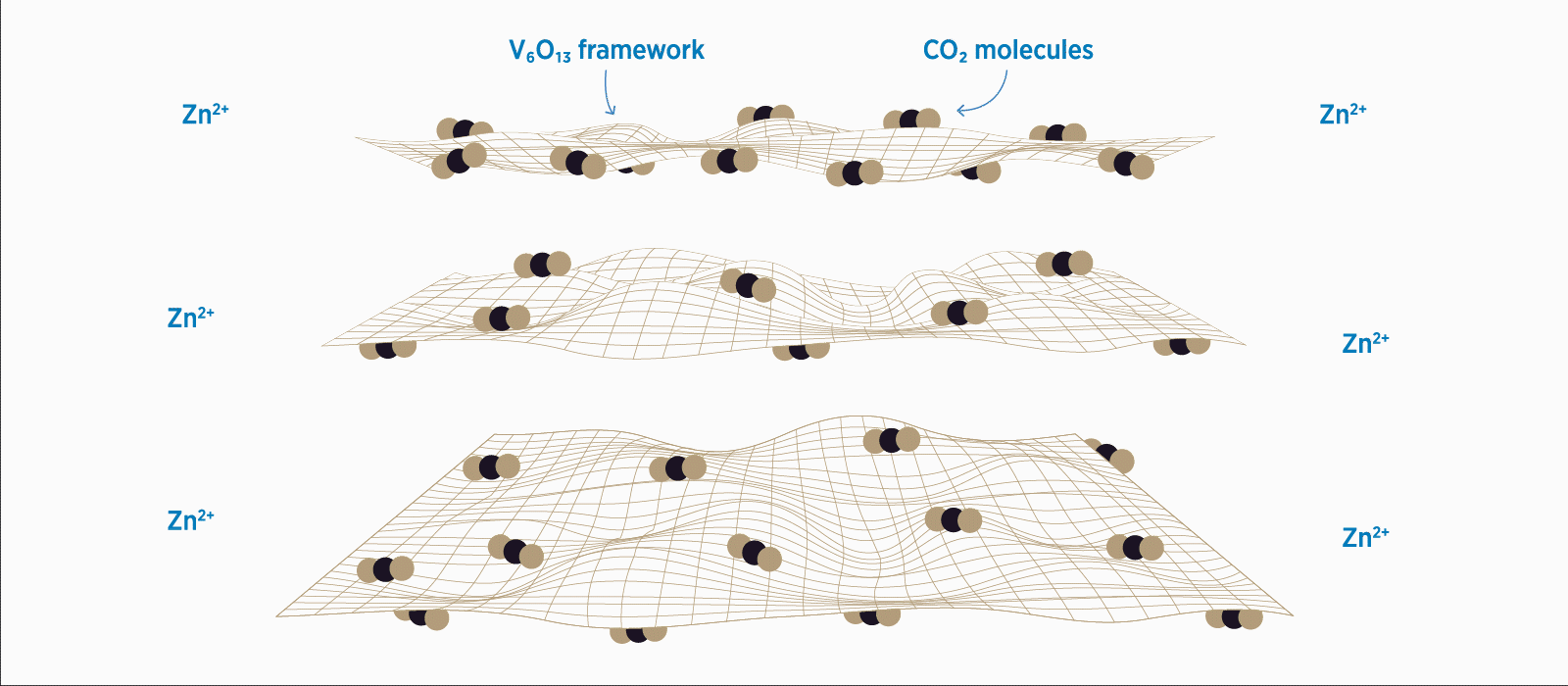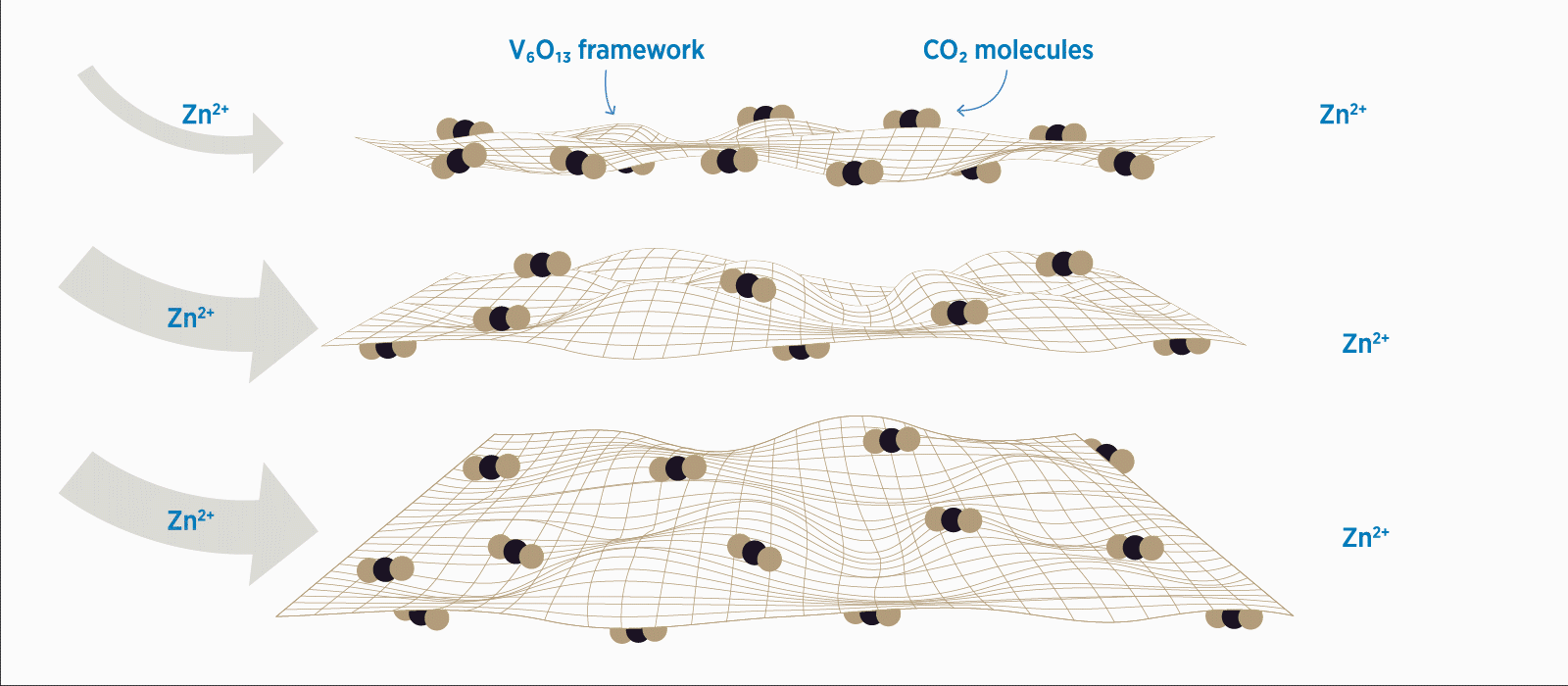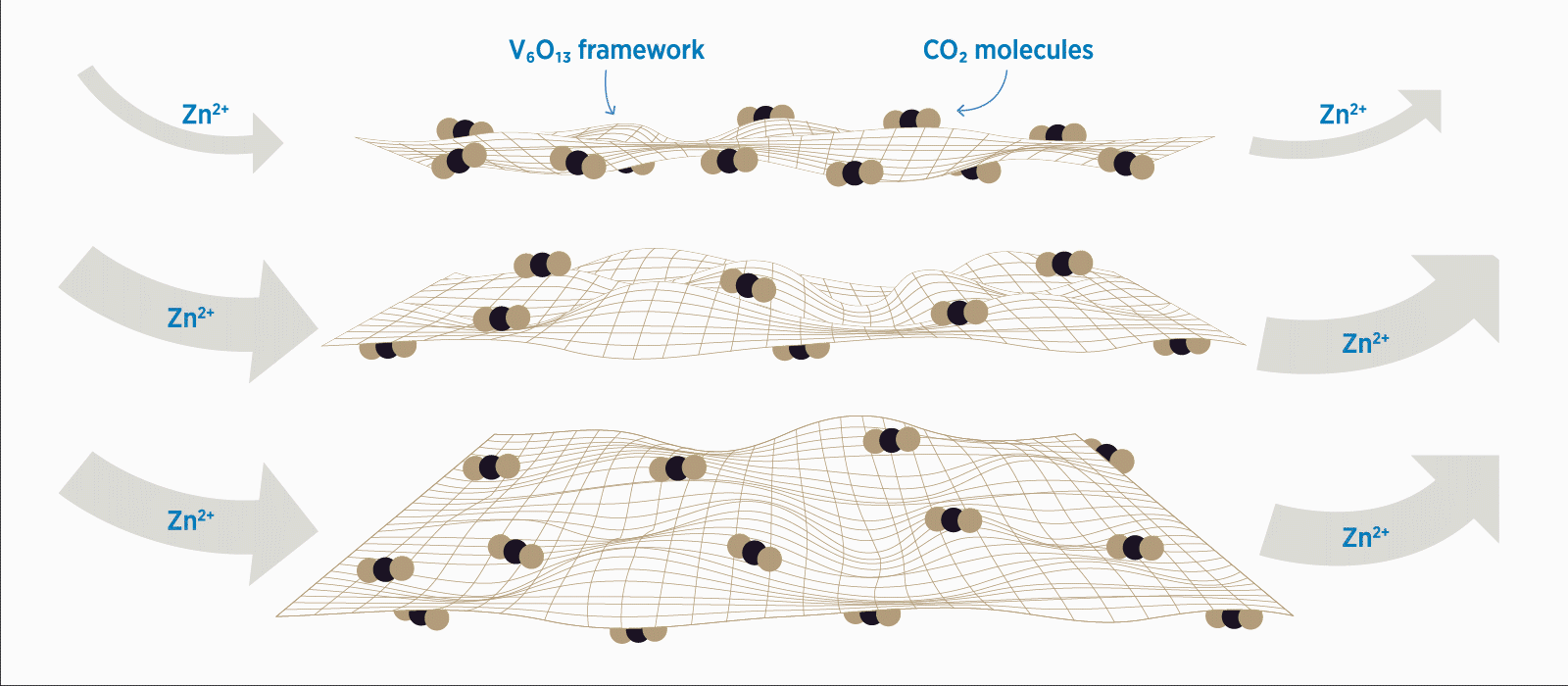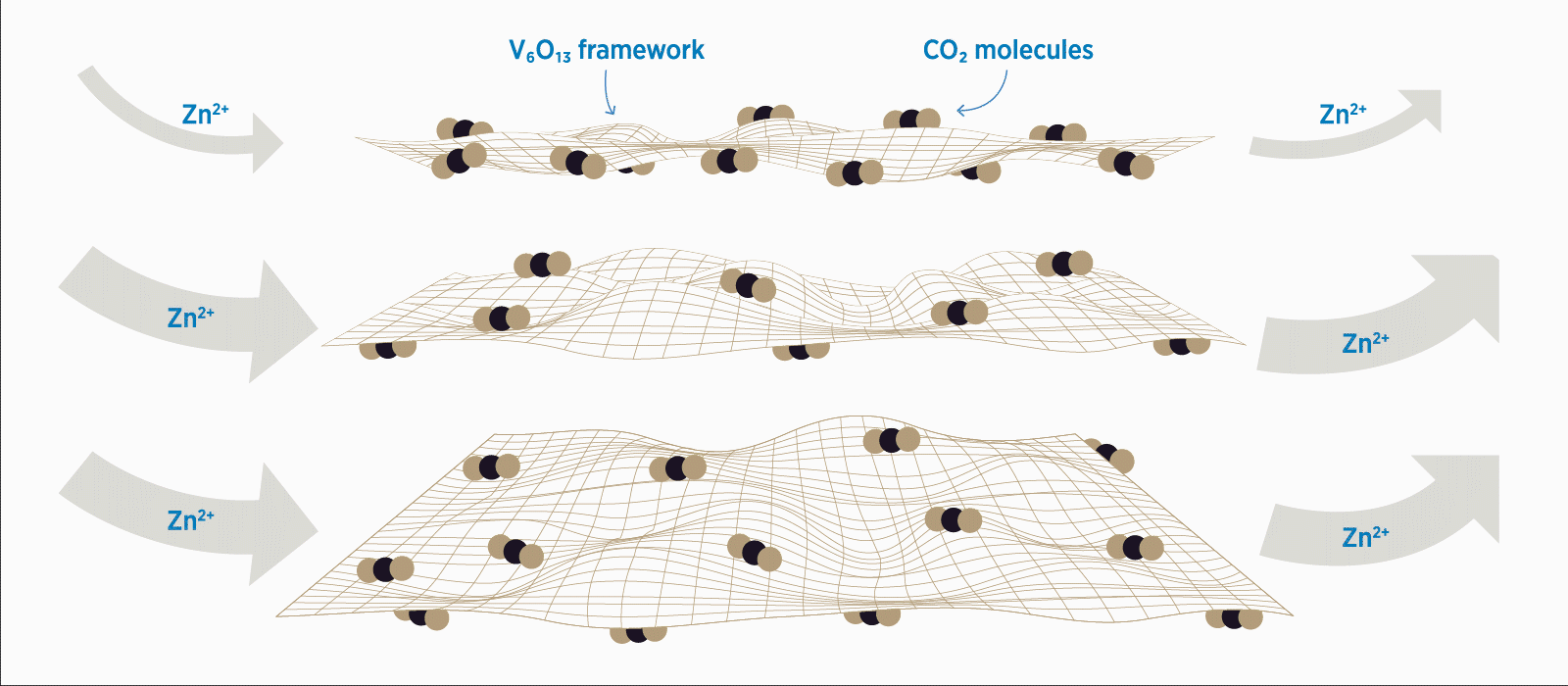
When the vanadium oxide framework is modified with CO2, zinc ions can easily diffuse across the material, giving zinc-ion batteries a greater ability to store charge.
© A*STAR Research
The study strongly supported the team’s hypothesis, yielding spectacular results: the addition of CO2 lowered the ZIB diffusion barrier over three times in one direction and by tenfold in the other.
This in turn gave the newly-designed ZIBs the ability to store over 40 percent more charge than their unmodified counterparts. Moreover, adding CO2 allowed the ZIBs to handle significantly more charge/recharge cycles, retaining a whopping 80 percent of its capacity after 4,000 cycles. In contrast, first-generation ZIBs begin to wind down to 58 percent capacity after just 570 cycles.
According to Yu, their results demonstrate how tweaking the chemical composition of ZIBs can help these batteries achieve higher energy densities, making them a viable contender against other battery technologies on the market. In the future, the team plans to explore a similar approach to enhancing other types of metal-ion batteries.
The A*STAR-affiliated researchers contributing to this research are from the Institute of High Performance Computing (IHPC).





