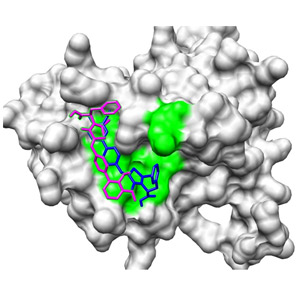
Fig. 1: After selection from an initial pool of molecules, the interaction of drug candidates (magenta and blue) with the BH3 binding site of Mc1-1A (green) can be predicted using a molecular docking program.
© 2010 P. Bernardo
When cell division begins to spin out of control, cellular suicide may represent the only means for preventing the onset of cancer. Accordingly, many tumors ensure self-preservation by the forced over-expression of proteins that block the controlled cell death mechanism known as apoptosis.
One of the families of proteins that are known to block apoptosis is Bcl-2. The chemotherapeutic agent ABT-737 effectively inhibits many Bcl-2 proteins, but is ineffective against another apoptosis-blocking protein Mcl-1, providing tumors with a ‘back door’ for drug resistance. “Mcl-1 is usually degraded much faster in cells, but in some cancer cells this degradation pathway is shut off,” explains Paul Bernardo from Christina Chai’s group at the A*STAR Institute of Chemical and Engineering Sciences in Singapore. “Compounds which can inhibit Mcl-1 would be valuable on their own or in combination therapy against cancers.”
In collaboration with the National University of Singapore, Chai’s team has now revealed a molecule that could offer the foundation for such a therapeutic strategy. They selected this compound from a library of 34 candidates containing structural elements of BH3I-1, a synthetic drug targeting the pro-apoptotic BH3 binding domain of Bcl-1 proteins, and sanguinarine and chelerythrine, two naturally occurring substances that bind and inhibit family member Bcl-XL.
The researchers identified two compounds with strong affinity for Mcl-1; one bound to both Bcl-XL and Mcl-1, while the other bound exclusively to Mcl-1. Although these two molecules are very similar, they contain differences in the relative positioning of two methoxy chemical groups that appear to have a significant impact on their binding specificity. “We were surprised that such subtle changes in the structure can affect the selectivity of these inhibitors,” says Chai.
Using computer modeling, Chai, Bernardo and their co-workers were able to predict how such seemingly minor differences could markedly alter the positioning and orientation of these two molecules within the Mcl-1 BH3 binding site (Fig. 1). This analysis also revealed how these same binding characteristics would physically exclude one compound—but not the other—from interacting effectively with Bcl-XL.
These structural data should also offer valuable guidance for the further optimization of this molecule. “Based on this work, we have synthesized some new derivatives that incorporate design features that are predicted to bind strongly and selectively,” says Bernardo. In parallel, the researchers are also partnering with colleagues in Singapore and the UK to characterize the anticancer potential of these new drug candidates. “We are now looking at the efficacy of these compounds in cells that over-express the Mcl-1 protein,” says Chai.
The A*STAR-affiliated researchers mentioned in this highlight are from the Institute of Chemical and Engineering Sciences, the Institute for Infocomm Research and the Experimental Therapeutics Center.



