As useful as X-rays are in modern medicine, astronomy and engineering, they can also be energy-intensive tools and invisible health hazards. To detect and visualise this form of ionising radiation, some devices make use of scintillators: special materials that, when hit by high-energy X-ray particles, absorb their energy and turn it into a vibrant glow.
Scintillators often contain rare elements with unique optical properties, known as lanthanides. However, most lanthanide-based scintillators only capture a small portion of energy from the original (primary) X-rays; the rest is often lost as heat or non-radiative decay, said Xiaogang Liu, an Adjunct Principal Scientist at the A*STAR Institute of Materials Research and Engineering (A*STAR IMRE).
“Lanthanide ions have trouble harvesting the full cascade of secondary X-ray energy that’s released after primary X-ray irradiation,” said Liu. “This inefficiency prompted us to rethink the energy transfer pathway between X-rays and these ions.”
Working with researchers from Xiamen University, Fujian Normal University and other institutes in China, Liu and A*STAR IMRE colleagues sought to improve this system through a mechanic called triplet exciton recycling.
“Excitons are bound pairs of electrons and holes that are generated when a material absorbs energy, such as from X-rays,” said Liu. “We theorised that triplet excitons—longer-lived than singlets, and often called ‘dark’ because they don’t emit light efficiently on their own—could act as efficient mediators to capture and redirect secondary X-ray energy to lanthanide cores.”
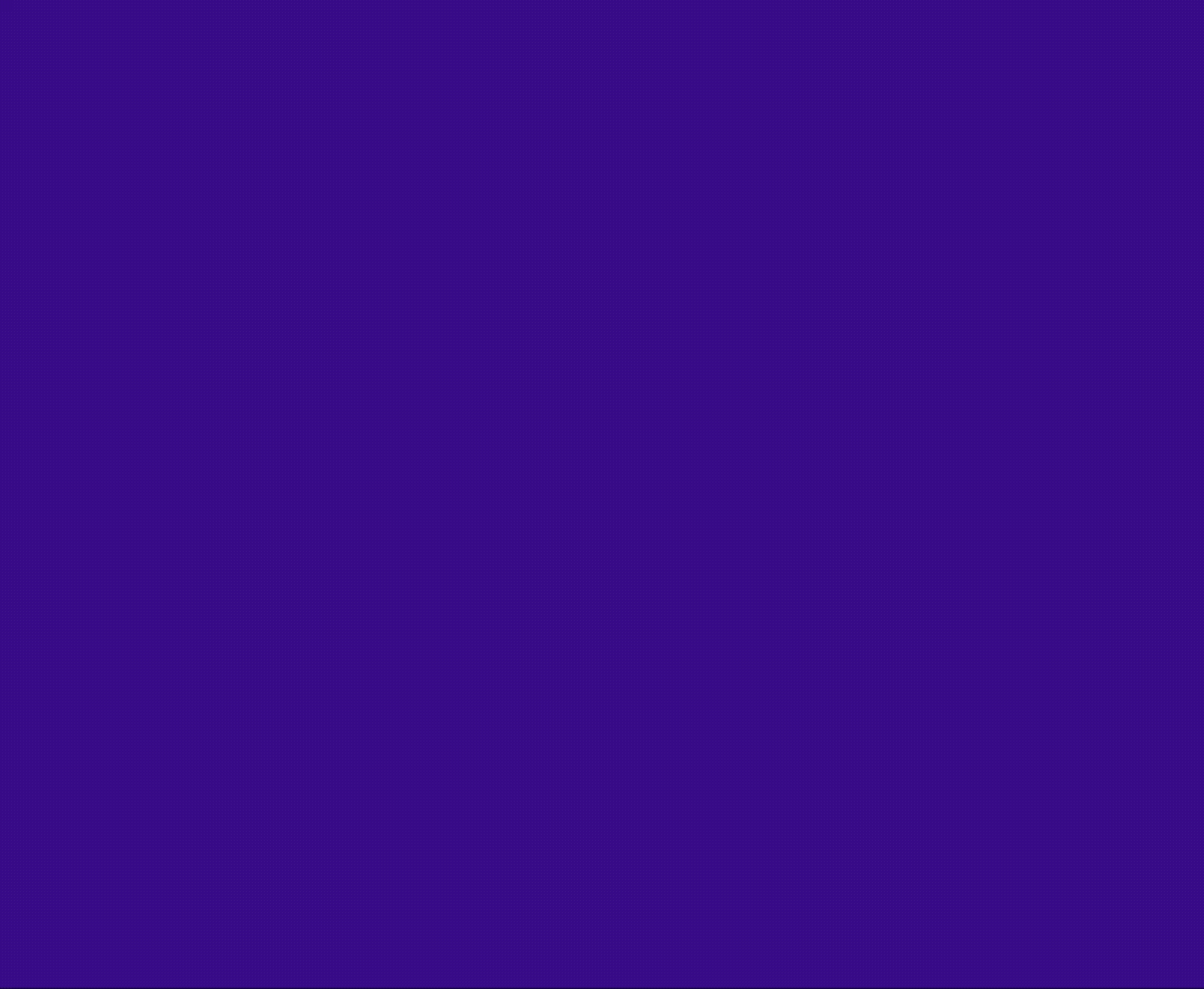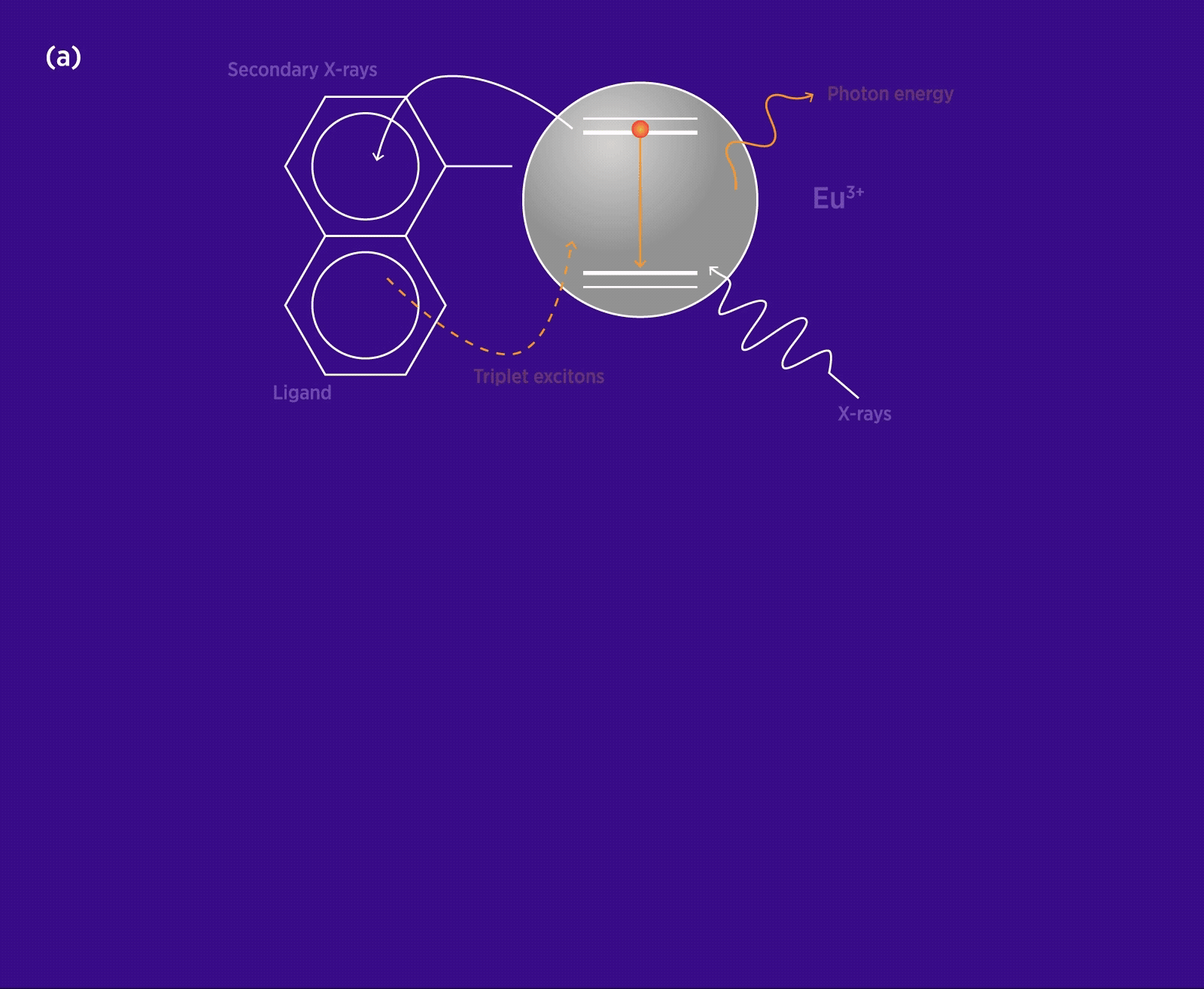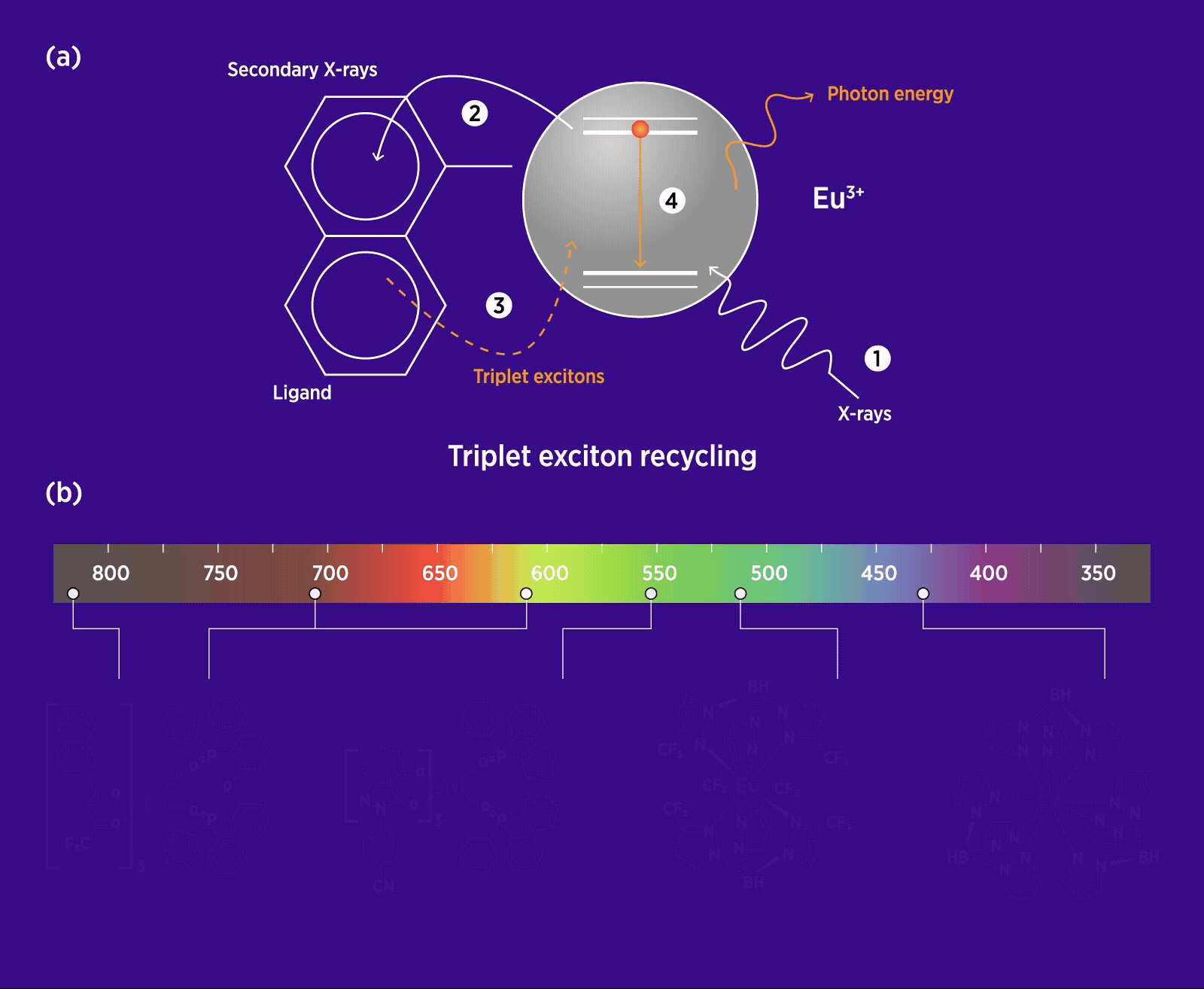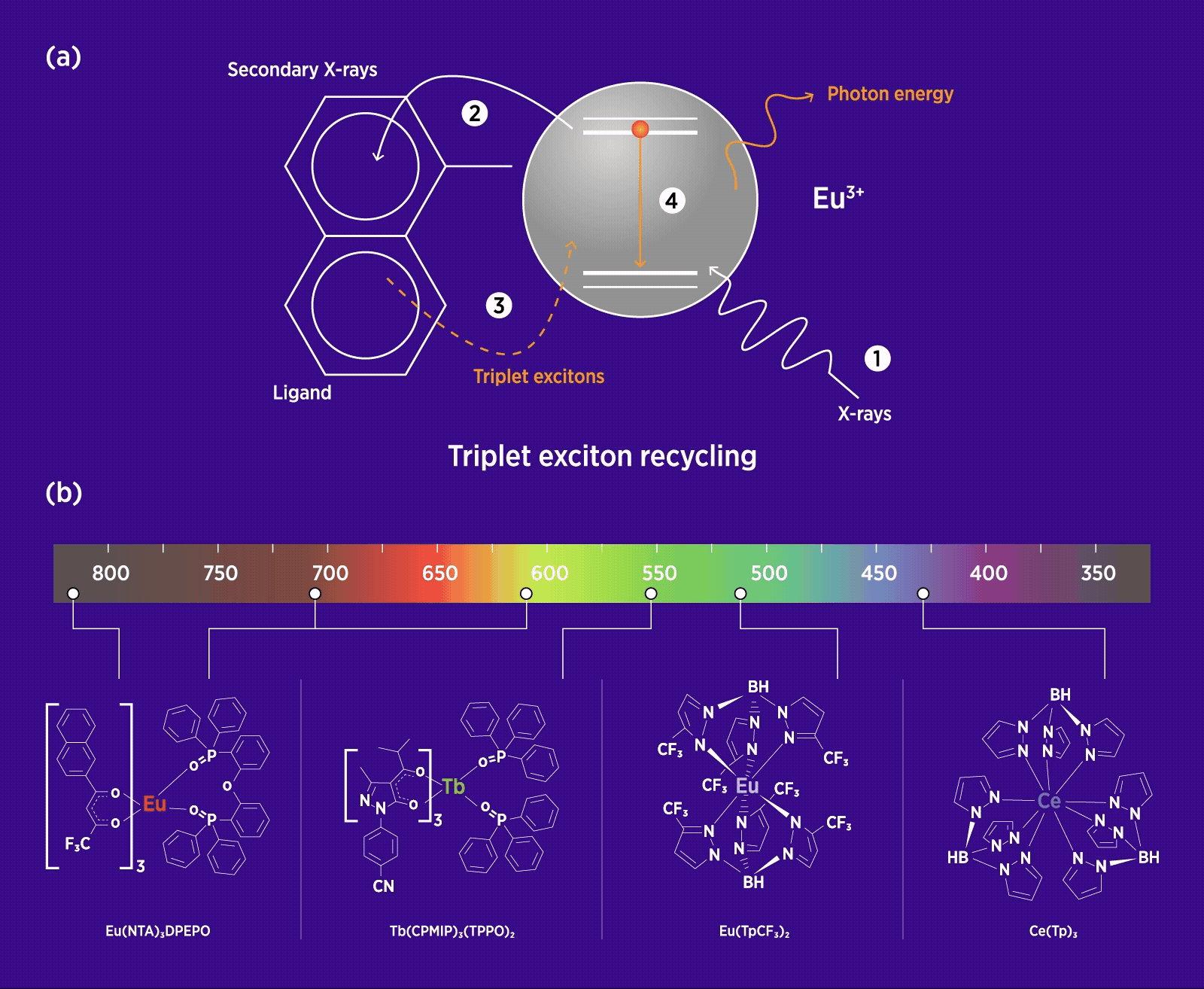
(a) Schematic of triplet exciton recycling in a europium (Eu)-based organolanthanide molecule, showing energy transfer from an organic ligand ‘antenna’ to its lanthanide centre. (1) X-ray irradiation triggers a cascade of secondary X-rays, which are (2) captured by the ligand, then (3) transferred back into the molecule. Once excited by the secondary X-rays, (4) hot charge carriers in the molecule relax, leading to the formation of optical excitons. (b) Molecular structures of four typical organolanthanide scintillators based on cerium (Ce), Eu and terbium (Tb), showing full-colour radioluminescence from ultraviolet to infrared light wavelengths. (Adapted from Xu et al. 2025)
© A*STAR Research
The team strategically designed and attached small organic molecules (ligands) to much larger scintillator molecules. Like radio antennae, these ligands were precisely tuned to capture waves of escaping secondary X-ray energy, convert them into dark excitons, then transfer the excitons to the scintillators’ lanthanide cores.
Compared to existing inorganic and organic scintillators, the team’s system proved to be several orders more efficient at emitting visible light. The team was also surprised by a stark performance difference when they tested their ligands with different lanthanide systems.
“We found f–f lanthanide complexes outdid d–f systems in efficiency despite the latter’s theoretically higher yields,” said Liu. “Our studies showed that f–f systems rely more on triplet exciton recycling, while d–f systems rely on direct energy capture by the luminescent centre.”
Liu added that these results highlight how strong light emissions don’t always translate to efficient scintillation, with exciton recycling instead playing a more decisive role.
Looking ahead, Liu noted that the team’s fully molecular-level system design could be a part of next-generation scintillators, optoelectronics and radiation detection platforms. The team hopes that their work may also enhance biomedical imaging probes and phototherapeutic systems.
“We’re improving our scintillator materials to be more compatible with in-body environments,” said Liu. “Our goal is to enable the uniform and efficient labelling of biological tissues for high-resolution molecular imaging, with minimal background interference.”
The A*STAR-affiliated researchers contributing to this research are from the A*STAR Institute of Materials Research and Engineering (A*STAR IMRE).





