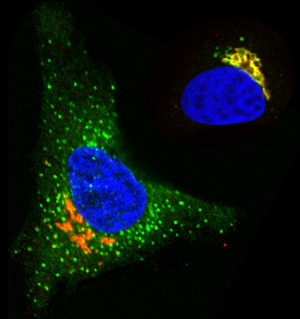
Fig. 1: In cells stimulated with EGF (left), but not control cells (right), O-glycosylation enzymes (green) are redistributed from the Golgi apparatus (orange) to the endoplasmic reticulum (blue, nucleus).
© 2010 F. Bard
Signaling proteins known as growth factors can induce changes in multiple aspects of cell physiology, including protein synthesis, cytoskeleton dynamics and adhesive properties. Some of these changes are regulated by enzymes called Src family kinases (SFKs), components of the signaling pathways activated by growth factor receptors.
Frederic Bard of the A*STAR Institute of Molecular and Cell Biology in Singapore and co-workers previously showed that SFKs are important for not only organizing the Golgi apparatus, a membrane-bound cellular organelle that processes newly synthesized proteins, but also regulating the movement of proteins through it. The exact role of SFKs at the Golgi apparatus, however, was unknown.
Bard’s team has now reported that Src regulates the Golgi chemical reaction called O-glycosylation, in which complex sugar molecules called glycans are added to proteins, and that this regulation involves the redistribution of GalNac-T enzymes, which catalyze the initial reaction.
The researchers examined the distribution of Golgi proteins in cultured cells treated with epidermal growth factor (EGF) or platelet-derived growth factor, and compared it to the distribution in untreated cells. They found that the growth factors cause several GalNac-Ts to be moved from the Golgi apparatus to the endoplasmic reticulum, another cellular organelle involved in protein synthesis (Fig. 1).
In another set of experiments, the researchers found that GalNac-T redistribution was blocked in cultured cells treated with EGF and an Src inhibitor, but not in cells treated with EGF alone, showing that the redistribution is dependent upon activation of Src by growth factor receptors.
Protein transport between the Golgi and endoplasmic reticulum involves the formation of vesicles that bud-off from one organelle before being moved to the other. This process is regulated by a protein called Arf1, and is dependent on the protein COP-I, which coats newly formed vesicles.
To investigate whether this process is required for GalNac-T redistribution, Bard’s team used a mutant form of Arf1, which blocks the final stages of vesicle formation. Protein movements from the Golgi apparatus were inhibited in cultured cells expressing the mutant, but not in cells expressing normal Arf1.
Finally, the researchers demonstrated that the GalNac-T redistribution induced by Src activation leads to a significant increase in cellular O-glycosylation.
“Src has been implicated in numerous cancers,” says Bard, “and one hypothesis is that its oncogenic effect is at least partly mediated by changes in adhesive properties through O-glycosylation of cell surface proteins. We are, therefore, currently investigating whether changes in O-glycosylation affect cell adhesion and migration.”
The A*STAR-affiliated researchers mentioned in this highlight are from the Institute of Molecular and Cell Biology.



