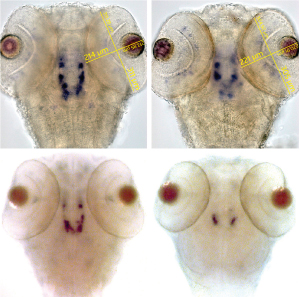
Fig. 1: Normal zebrafish embryos (left) have more dopamine-producing neurons than embryos lacking the WD40 domain of LRRK2 (right), as revealed by staining for tyrosine hydroxylase (top) and dopamine transporter (bottom).
Reproduced from Ref. 1 © 2010 J. Liu
Parkinson’s disease (PD) is a progressive neurodegenerative condition characterized by tremor and rigidity, which result from the death of dopamine-producing neurons in the midbrain.
The disease is associated with mutations in at least five different genes, including several in different regions of the gene encoding an enzyme called Leucine-rich repeat kinase 2 (LRRK2). Some mutations of this protein block its activity while others enhance it, and neuroscientists consider it as a master regulator that is critical for PD development. However, LRRK2’s biological functions—thought to be many—are largely unknown.
To investigate, a group of researchers led by Jianjun Liu, associate director and senior group leader at A*STAR’s Genome Institute of Singapore isolated the zebrafish version of the LRRK2 gene. They used a method called RNA inhibition to block synthesis of the LRRK2 protein in the zebrafish, and found this to be lethal to the embryos.
The researchers then used a variation of the same method to interfere with LRRK2 synthesis, so that the embryos made a shortened version of the protein lacking a region called the WD40 domain. These embryos developed normally, but there was increased death of dopaminergic cells (Fig. 1), and some of the brain’s neural pathways were disorganized. As a result, the larvae were unable to swim properly.
Liu and his co-workers also found that these defects could be partially rescued by injections of normal zebrafish and human LRRK2, and by mutant forms of zebrafish LRRK2 containing the WD40 domain. The swimming defects, but not cell death, were also rescued by L-Dopa, the dopamine precursor commonly used to treat PD patients.
By showing that deletion of the WD40 domain from zebrafish LRRK2 is sufficient to produce neurodegeneration and symptoms that are very similar to PD in humans, the team’s results provide fresh insights into LRRK2 function. Not only do the results underline the usefulness of the zebrafish as an animal model of human PD, they also indicate that these fish could be used to screen new drugs developed to treat PD, according to the team.
“It is hard to tell how the WD40 deletion leads to Parkinson’s-like degeneration and symptoms,” says Liu, “but we are now generating stable mutant zebrafish lines to address this issue. The WD40 domain is involved in protein–protein interactions, so we speculate that deleting it could interrupt the interaction between LRRK2 and its substrate proteins.”
The A*STAR-affiliated researchers mentioned in this highlight are from the Genome Institute of Singapore.



