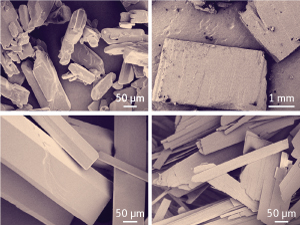
Fig. 1: Scanning electron microscopy images of Tolbutamide polymorphs Form I (top left), Form II (top right), Form III (bottom left), and (bottom right) Form IV.
Reproduced, with permission, from Ref. 1 © 2010 Wiley-Liss, Inc.
A common drug called Tolbutamide that is used worldwide in pill form to treat diabetes is not being manufactured in its most stable form, according to a team of researchers led by Reginald Tan at the A*STAR Institute of Chemical and Engineering Sciences, Singapore. This unexpected finding means that during the manufacturing process, Tolbutamide could transform into another, more stable form with different characteristics. Since such transformations are not normally condoned by regulatory agencies, the team’s results could be used to develop a more efficient and robust manufacturing process for Tolbutamide.
The chemical structure of Tolbutamide includes a rigid benzene ring from which a chain protrudes. Each of the seven bonds in the chain can rotate freely, which means that the molecule is polymorphic—it can assume many different shapes. Consequently, it crystallizes in several different forms depending on which of the shapes are packing together. The different crystals display different physical properties, such as melting points. Four standard crystal forms (I to IV) (Fig. 1) were established in the 1970s, but discrepancies as to the stability of individual crystals emerged at the time between different researchers. More recent work has shown that Form I transforms between two different crystals depending on temperature.
Tan and his co-workers decided to re-analyze the polymorphic crystal forms of the drug using state-of-the-art equipment and techniques. These included single crystal X-ray diffraction, powder X-ray diffraction, solid-state nuclear magnetic resonance, scanning electron microscopy, hot-stage microscopy, Fourier transform infrared spectroscopy and differential scanning calorimetry.
Their work not only resolved the former discrepancies, but revealed that the most stable form is actually Form II, a spiky, needle-like form that is difficult to process industrially. The industrial formulations of the drug found in pills are typically of Form I, and this is still the most desirable form for manufacturing, Tan says. But the published work has now defined a risk that Form I could transform into Form II during the manufacturing process.
With its newly obtained data, the research team can now design much better strategies for producing Form I of the drug in a robust and assured way. The team is investigating crystallization using process analytical technology (PAT), which combines the latest in sensors and control technology and is expected to lead to a more automated and robust manufacturing process.
“We are also working on particular forms called co-crystals—two molecules crystallizing into the same crystal structure,” he says. “These may or may not be polymorphic.”
The A*STAR-affiliated researchers mentioned in this highlight are from the Institute of Chemical and Engineering Sciences.



