Much like how a maestro conducts the various sections of an orchestra, pancreatic stellate cells (PSCs) can direct the behaviour of multiple cell types in a form of cancer called pancreatic ductal adenocarcinoma (PDAC). These PSCs, which become cancer-associated fibroblasts (CAFs) when activated, are known to influence the activity of natural killer (NK) cells, which are immune cells that can kill tumour cells.
However, the exact interactions between PSCs and NK cells are not so clearly defined, with past research providing conflicting information. “One study reported NK cell killing of PSCs, whilst another showed that PSCs impaired NK cell function,” explained Group Leader Joe Yeong and Postdoctoral Researcher Rachel Fincham from the A*STAR Institute of Molecular and Cell Biology (A*STAR IMCB). “We wanted to better understand this relationship to find interactions that could be relevant for patient stratification or therapeutic targeting.”
Yeong’s team collaborated with researchers from Queen Mary University of London in the UK to investigate the bidirectional interactions between NK cells and PSCs in PDAC.
By growing PSCs and NK cells together in a co-culture system, they observed that PSCs were transformed into a more activated state and had higher expression of a protein marker for anti-tumour immunity. Notably, such activation only happened when PSCs and NK cells were in direct contact with one another.
In parallel, the NK cells were changing, too. Elevated levels of key proteins as well as increased release of cytokines and chemokines suggested that the NK cells were being primed, modifying their anti-tumour behaviour.
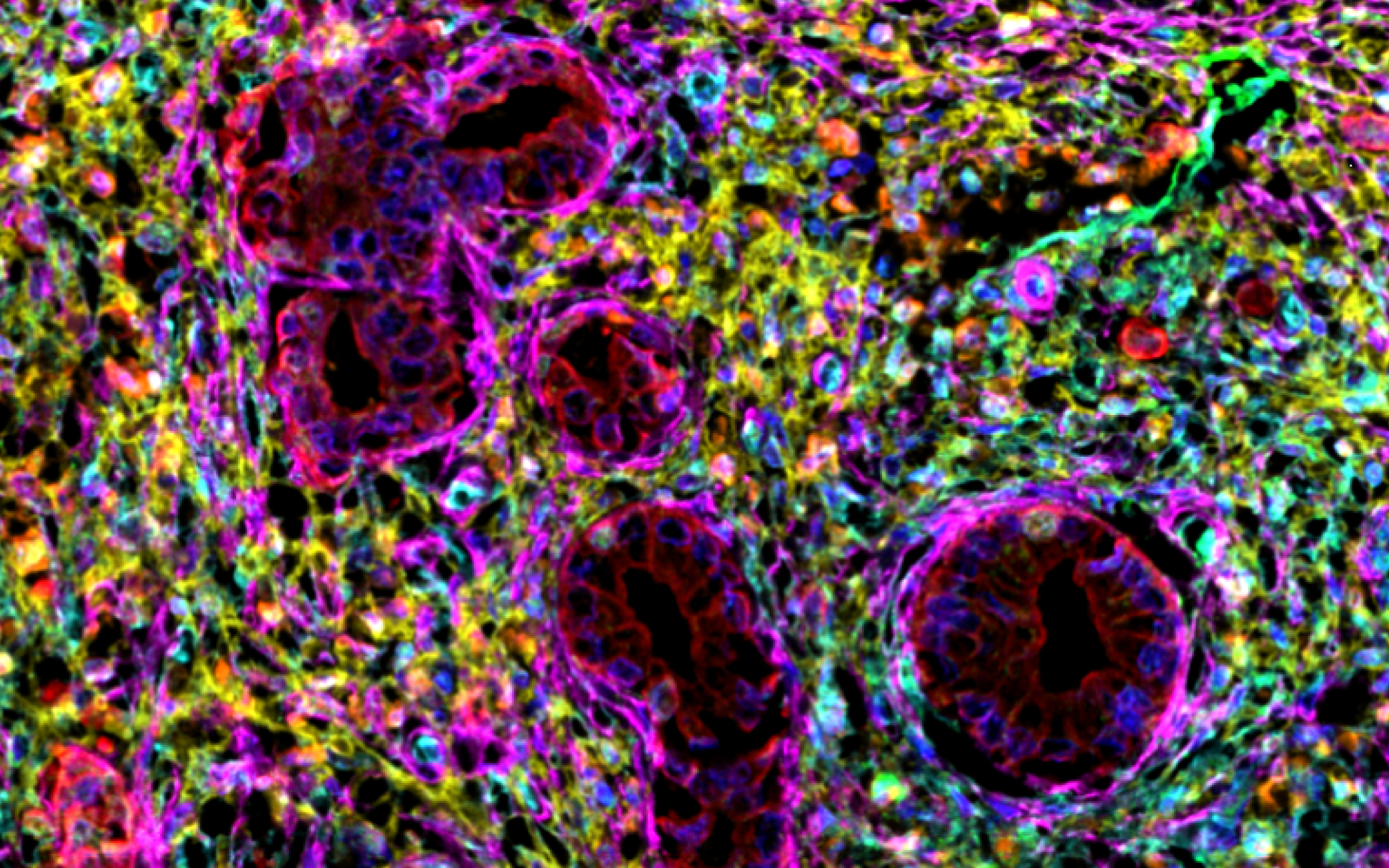
Looking into patient data, the team found that the amount of NK cells infiltrating into the tumour microenvironment (TME) could not reliably predict differences in survival outcomes. This striking discovery was achieved using multiplex immunohistochemistry, an imaging technique that enables labelling of multiple protein markers of interest on the same sample even over repeated runs.
This led the researchers to evaluate cellular spatial proximity, which revealed that it was not the total number of NK cells within a tumour, but their distance to the PSC/CAFs that differentiated PDAC patients with worse prognosis compared to those who survived for more than 30 months post-diagnosis. When NK cells were closer to PSC/CAFs, patients tended to have better survival outcomes.
“Our work highlights the importance of spatial biology in uncovering critical interactions within the TME and suggests the potential for patient stratification based on NK-PSC/CAF proximity,” said Yeong and Fincham. Moreover, these parameters could be used to identify more appropriate treatment plans for short versus long-term PDAC survivors.
To delve deeper into these NK-PSC/CAF interactions, Yeong and team are now turning to even higher-throughput imaging techniques combined with spatial omics, with the hopes of uncovering new therapeutic targets for pancreatic cancer.
The A*STAR-affiliated researchers contributing to this research are from the A*STAR Institute of Molecular and Cell Biology (A*STAR IMCB).