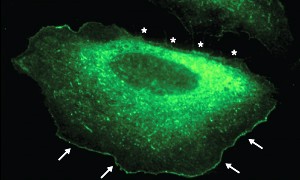
Fig. 1: In migrating cells, fluorescently labeled IRSp53 is localized at the leading edge (arrows) but is absent from areas that are retreating (stars).
© 2010 E. Manser
Cells stay in touch with their surroundings via the extension of filopodia—slender membrane appendages that facilitate migration but which are also thought to contribute to the detection of chemical signals from the environment.
Filopodium formation depends on the choreographed reorganization of filament-forming actin molecules within peripheral cellular structures known as lamellipodia, and new research by a team led by Ed Manser from the A*STAR Institute of Medical Biology in Singapore has revealed important details about the regulation of this process.
Manser and his co-workers found evidence that IRSp53, a protein that helps promote filopodial outgrowth, interacts with the 14-3-3 family of regulatory factors. This association requires the presence of a pair of specific chemical modifications on IRSp53, and the formation of this complex physically prevents IRSp53 from associating with other proteins that contribute to filopodia formation. For example, 14-3-3 binding appears to mask the SH3 protein domain that mediates interaction with the Eps8 protein.
IRSp53 normally localizes into a narrow strip along the ‘leading edge’ of cells, the segment of the plasma membrane from which migration is initiated (Fig. 1). In the absence of the SH3 domain, however, this localization is disrupted, considerably inhibiting—but not entirely preventing—filopodium formation. Remarkably, the substitution of an SH3 domain from another protein, IRTKS, failed to restore normal localization. “We were surprised to find that the SH3 domain was the most important determinant of how this protein finds its way to the cell edge, and that the SH3 domain from a highly related protein did not work,” says Manser. Subsequent experiments further highlighted this specificity by demonstrating that the IRTKS SH3 lacks the ability to bind Eps8, an interaction that appears to be crucial for attracting IRSp53 to the cellular leading edge.
Cells over-expressing an IRSp53 variant that lacks the motifs necessary for 14-3-3 binding produced especially elongated and branched filopodia. These cells did not exhibit greater numbers of filopodia than those expressing normal IRSp53, but instead produced filopodia that lasted significantly longer, suggesting that 14-3-3 selectively disrupts the IRSp53-Eps8 interaction in a manner that promotes retraction of filopodia rather than blocking their initial formation.
These findings stand in contrast to previous models, and help resolve a debate in the cell biology community. “Other studies have suggested that a membrane-binding domain at the front of IRSp53 was the key to localizing this protein,” says Manser. “Our paper now establishes how cells turn this protein ‘on’ and ‘off’ at the cell edge.”
The A*STAR-affiliated researchers mentioned in this highlight are from the Institute of Medical Biology and the Institute of Molecular and Cell Biology.



