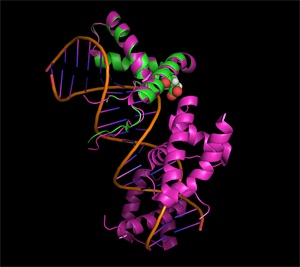
Sox17, with the critical amino acid residue highlighted in red, induces cells to revert to an undifferentiated state when mutated and expressed with Oct4.
Induced pluripotent stem cells (iPSCs) are undifferentiated cells that retain the ability to become any cell type in the body. Several proteins have the ability to revert differentiated cells back to this undifferentiated state, but the biochemical mechanisms that enable these proteins to do so has been unclear.
Prasanna Kolatkar at the A*STAR Genome Institute of Singapore and co-workers have now identified a structural motif that gives proteins the ability to reprogram mature cells into an undifferentiated pluripotent state. The researchers examined two transcription factors called Sox2 and Sox 17, which closely resemble each other and bind to the same DNA sequences, but which perform very different functions during development.
Sox2 interacts with another transcription factor called Oct4, and this interaction is required for the development of an embryonic structure called the epiblast. Sox17 also interacts with Oct4, but this leads to the differentiation of endoderm, which produces the gut. Sox2 induces pluripotency when expressed in embryonic stem cells, whereas Sox17 drives them to differentiate into endoderm-like cells.
Kolatkar and his co-workers set out to investigate whether Sox2 and Sox17 compete with each other to bind to Oct4, and also to determine whether the different outcomes of their interactions with Oct4 can be explained by differences in structure. They performed computational analyses to establish the configuration of the Sox2/Oct4 binding sites, and then used genetic engineering to produce mutated versions of Sox2 and Sox17 in which a single amino acid in the binding site is substituted for another.
Their analysis revealed that a single amino acid substitution at a specific site in either of the Sox proteins switches its preference for interacting with Oct4 on co-binding sites differing by one base pair. Significantly, these single point mutations were sufficient to cause Sox2 and Sox17 to switch their biological activity. For example, mutated Sox17 (pictured) could induce cells to revert to a pluripotent state when expressed with Oct4, whereas mutated Sox2 could induce endoderm differentiation but not pluripotency.
One possible reason behind the change in biological activity is that mutant Sox17 can interact with Oct4 to activate pluripotency genes, while mutant Sox2 cannot. “This is true synthetic biology in the sense that we are making artificial proteins that can enhance specific properties or functions,” says Kolatkar. “We are pinpointing the exact DNA sequences that different protein combinations recognize.” The researchers are now testing other residues and protein partners to see if they can result in better iPSC generation.
The A*STAR-affiliated researchers contributing to this research are from the Genome Institute of Singapore.



