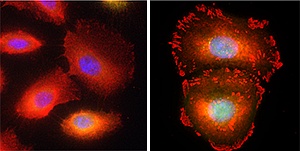
TAp63β-mediated focal adhesion in Caski cells, with the focal adhesion protein vinculin in red and DNA in blue
Human papillomavirus (HPV) is known to be the primary causative agent for cervical cancer. The virus exerts its harmful effects by expressing a cohort of proteins — most notably E6 and E7 — to actively sabotage the host cell’s regulatory machinery. The disruption may affect numerous pathways involved in cellular growth and division.
Françoise Thierry and co-workers at the A*STAR Institute of Medical Biology’s Papillomavirus Regulation and Cancer research group have now found that HPV also causes tightly-anchored cells to become unmoored from their environment as a prelude to tumor growth. They have identified the cellular protein p63 (also known as transformation-related protein 63) as a key factor in this process.
“Many cellular genes belonging to adhesion pathways are modulated in cervical carcinoma cells expressing the E6 and E7 HPV oncogenes,” says Thierry. However, p63 is a complex target. The gene encoding p63 can produce not one but six different variants (isoforms) of the same protein that differ in size and structure. Some isoforms have an activation domain at one end (TA), while others (ΔN) lack this domain. Although the functions of these isoforms have not been fully elaborated, previous studies have linked ΔNp63α to certain skin cancers.
Thierry and her team worked with Caski cells, a cervical cancer-derived epithelial cell line that expresses HPV E6 and E7. They identified hundreds of genes whose expression was modified by E6 or E7, 123 of which were likely targets of p63 regulation. Significantly, many of these genes are known to contribute to cellular adhesion. This finding came as a surprise because the effect appeared to be mediated by the TAp63β isoform. “Based on previously published work, we expected that one of the ΔNp63 isoforms would be the major player in the epithelium rather than TAp63,” says Thierry.
In subsequent experiments, Thierry and her team showed that overexpression of TAp63β in Caski cells counteracted the abnormal gene regulation associated with E6/E7 expression. The researchers learned that E6 in particular induces accelerated degradation of TAp63β, leading to reduced adhesion. Conversely, overexpression of TAp63β in cervical cancer cells results in increased formation of dense cellular clusters called focal adhesions, with elevated production of key adhesion proteins (see image).
Thierry plans to use this work as a springboard to further explore the relative contributions of the various p63 isoforms in normal epithelial function, and to examine the role of TAp63β in tumor development. “We would like to demonstrate that TAp63 is expressed in tumor stem cells in our system and that its regulation by E6 is an essential step in neoplastic progression,” says Thierry.
The A*STAR-affiliated researchers contributing to this research are from the Institute of Medical Biology.



