When a virus enters a cell, it hijacks the cell’s functions and turns it into a factory that produces more viruses. Dendritic cells (DCs)—a type of white blood cell in the body—play a crucial role in activating other cells in the immune system to contain the infection.
To accomplish their mission, DCs engulf immune complexes which are made up of antibodies bound to an invading virus. The immune complexes are then ‘cut up’ and displayed on the surface of DCs in a process known as antigen cross-presentation.
Subsequently, another subset of immune cells called T cells encounter these antigen-presenting DCs and become activated, maturing into killer T cells that hunt down virus-infected cells. Enhancing DC function could therefore lead to better clearance of infections.
In this study, researchers from A*STAR’s Institute of Molecular and Cell Biology (IMCB) and Singapore Immunology Network (SIgN), led by Cheng-I Wang, focused on how a protein called TRIM21 mediates the immune response to adenoviruses, a class of viruses that can cause conjunctivitis or gastroenteritis, among other health conditions. They focused on TRIM21 because the protein is involved in the degradation of the immune complexes and mediates signaling inside DCs.
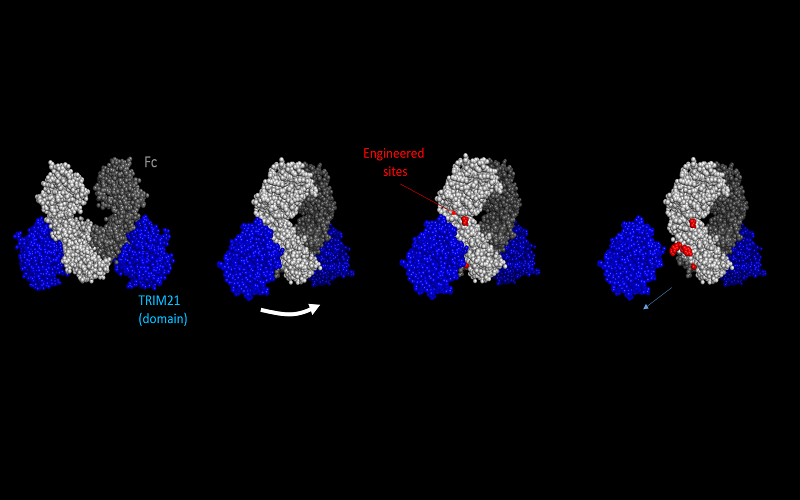
“TRIM21 binds to a segment known as the Fc region of the antibody in immune complexes. We hypothesized that a more stable TRIM21-Fc complex in DCs may result in stronger immune responses,” said Wang.
Although the structure of the TRIM21-Fc complex is known, mutations in the Fc region that would enhance TRIM21-binding could not be predicted. Hence, Wang’s team generated two billion antibody variants with mutations in the Fc/TRIM21 interface. This allowed them to isolate an antibody that has 100 times higher affinity to TRIM21 than typical antibodies.
The researchers demonstrated that their high-affinity antibody enhanced DC activation and maturation, which in turn resulted in a significant expansion of killer T cell populations targeting adenovirus-infected cells. They noted that stronger TRIM21 binding antibodies may have profound implications for the development of safer and more effective vaccines that rely on weakened live viruses to generate an immune response from the body.
“Our vaccine, like recombinant or subunit vaccines, will be safer. At the same time, it can induce strong immune responses through enhanced Fc-TRIM21 interaction,” said Wang.
“Going forward, we want to verify if our findings in vitro remain true in vivo. Using mouse models of human disease, we will be testing viral and cancer vaccines that carry antigens to DCs via Fc-engineered antibodies,” he said.
The A*STAR-affiliated researchers contributing to this research are from the Institute of Molecular and Cell Biology (IMCB) and the Singapore Immunology Network (SIgN).