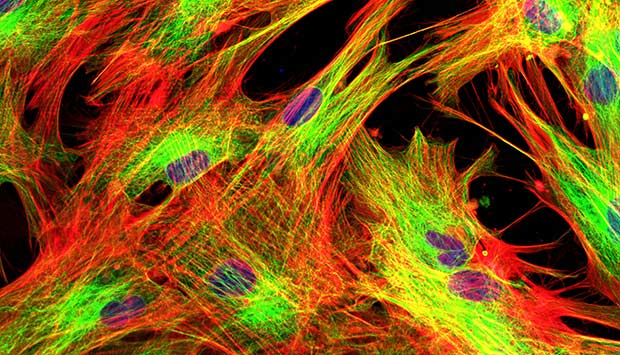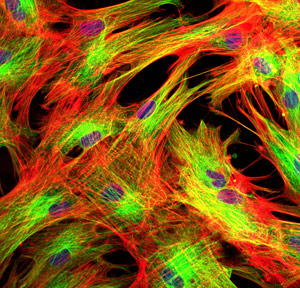
Skin cells were subjected to stress so the researchers could examine the cellular pathways of the stress response.
© vshivkova/iStock/Thinkstock
The classes of RNA molecules encoded by DNA sequences previously considered non functional may play a vital role in cell stress responses, and could one day lead to cancer treatments. A*STAR researchers have identified a class of the long non-coding RNAs responding upon oxidation stress and characterized and specified their functions across stress stimuli and cell types.
Human skin and lung cells were subjected to oxidative stress, by the research team, stimulating cellular pathways of repair and survival. While protein-coding genes were generally inhibited, stress caused noncoding genomic regions to produce thousands of RNA molecules called ‘long noncoding RNAs (lncRNAs)’.
Author Igor Kurochkin says the role of lncRNAs is a mystery. “We don’t know their function, or even if they function at all.” They may act in the evolution of new genes; in helping cells respond to stress; in interaction with genes or proteins; or, says another author, Vladimir Kuznetsov, “all of these at once!”
Unexpectedly, lncRNAs were found to accumulate at structures, known as polysomes, where proteins are assembled. According to Kuznetsov, “it’s possible that lncRNAs interfere with protein production at polysomes.”
He finds this interaction fascinating: “The cross-regulation of coding and noncoding RNAs lies at the intersection of many questions and may provide a major mechanism for evolutionary adaptation”, says Kuznetsov. “The field of studying protein-RNA interactions is huge and growing fast.”
The researchers, from several disciplines, could not exclude the intriguing possibility that lncRNAs actually code for proteins. Certainly some encode smaller molecules, known as polypeptides, made from the same building blocks as proteins. Many of these polypeptides are functional, for instance as hormones. The team now intends to study their role in combating oxidative stress. Kurochkin describes this as “a work in progress”.
The implications of these findings can be linked to medical conditions from chronic stress to neurodegenerative diseases. Some types of lncRNAs are common in cancer cells and could be a direct target for treatment. Kuznetsov explains: “Our study is very simple because it uses well-organized cells. In cancer, this organization is disrupted, unregulated, but the same machinery is at work.” Kurochkin agrees. “We need first to develop more complex experimental designs, models and interpretations,” he says. “Eventually we’ll learn how to manipulate it.”
Their research turns on its head the whole concept of noncoding DNA. In fact, Kuznetsov says, “There is no ‘junk DNA’. At least 80 per cent of the human genome initiates RNA production. RNA, DNA and proteins are now equal partners, we just don’t yet understand their complementary roles.”
The A*STAR-affiliated researchers contributing to this research are from the Bioinformatics Institute, the Institute of Molecular and Cell Biology and the Institute of Medical Biology. For more information about the team’s research, please visit the Genome and Gene Expression Data Analysis Division webpage.