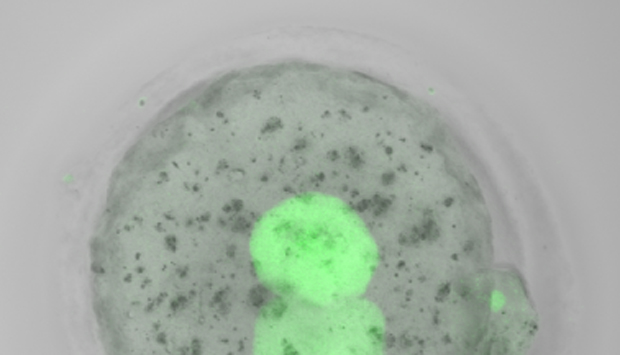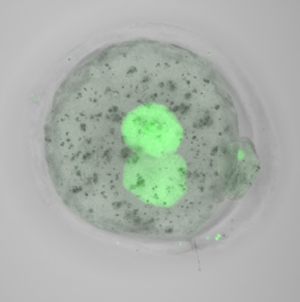
Normal levels of functional TRIM28 protein (green) are detectable within an hour after transplanting newly fertilized TRIM28-deficient nuclei into normal embryos that lack nuclei.
© 2013 AAAS
Chromosomes are heavily adorned with methyl chemical groups that alter the activity of nearby genes. The parental chromosomes, contributed by sperm and egg, display distinctly different methylation patterns and most modifications are stripped away shortly after fertilization. However, a subset of these ‘imprints’ is protected. Now, a sophisticated technique for single-cell analysis has broadened the understanding of this process1.
Daniel Messerschmidt’s team at the A*STAR Institute of Medical Biology in Singapore previously identified a protein called TRIM28 that binds certain methylated sites and recruits enzymes that preserve the appropriate imprinting pattern2. They noted that individual mouse embryos lacking the TRIM28 gene showed highly variable developmental abnormalities, an observation they could not fully explain at the time. “Our hypothesis was that these embryos were ‘mosaics’ in which different genes were affected, causing different defects in individual cells,” says Messerschmidt. “Thus, the embryo as a whole would always display a unique phenotype.”
To test this model, Messerschmidt’s team partnered with William Burkholder and co-workers at the A*STAR Institute of Molecular and Cell Biology, who had developed an assay that could be performed within a sort of ‘microchip’ to analyze the chromosomal methylation patterns of individual embryonic cells.
In the earliest days of the embryo, new proteins are generated entirely from maternally produced RNAs contained within the egg, with embryonic genes only taking control later in development. The researchers therefore generated genetically modified female mice whose eggs lacked TRIM28, so that they could better characterize the associated protein’s post-fertilization role. Their analysis of six genetic regions revealed that normal embryonic imprinting patterns are disrupted when maternal TRIM28 is absent. Importantly, the specific affected regions varied between cells, confirming Messerschmidt’s mosaic hypothesis. Although several imprints remained intact in any given cell, the cumulative effect is fatal to the embryo. “We only examined 6 of 25-plus imprinted regions,” says Messerschmidt. “The lethal phenotype results from the widespread effect over these many loci.”
Remarkably, the researchers could recover a modest percentage of normally imprinted embryos by transplanting genetic material from newly fertilized, TRIM28-deficient embryos into healthy embryos that contained normal levels of maternal TRIM28 protein (see image). These developed into healthy, fertile mice. Given that similar defects in imprinting maintenance have been observed in human diseases, Burkholder believes these findings could aid early-stage diagnostics and even treatment. “We are exploring the possibility of adapting our assay for humans, and testing in vitro-fertilized embryo biopsies for imprinting defects is one idea we have in mind,” he says.
The A*STAR-affiliated researchers contributing to this research are from the Singapore-based Institute of Medical Biology, Institute of Molecular and Cell Biology and Genome Institute of Singapore.