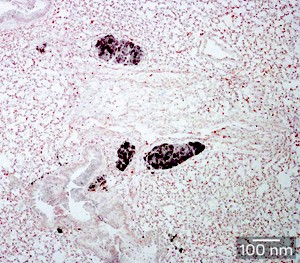
Fig. 1: Staining for cancer-specific antigen reveals colonies of disseminated tumor cells (dark spots) within the lungs of a 5-week-old RET.AAD mouse.
© 2010 J.-P. Abastado
Traditional models of cancer progression assume that cancer growth spreads to sites throughout the body only after the primary tumor has had ample opportunity to develop. Many scientists have reported data that challenge this principle, however, and the timing of metastasis remains a subject of controversy.
“Studies performed in the 1950s, which measured the growth rate of human tumors, led to the proposal that metastasis must be initiated long before the primary tumor is diagnosed,” says Jean-Pierre Abastado of the A*STAR Singapore Immunology Network (SIgN). More recent findings further support this hypothesis, he adds, “although not everybody agrees with this model.”
Nevertheless, Abastado and his co-workers at SIgN recently demonstrated striking evidence for early migration of cancer cells in RET.AAD mice, a melanoma model that initially exhibits tumor formation in the eye but soon develops metastases throughout the body. The researchers were analyzing genomic mutations within these various tumors to determine whether these non-ocular sites represent late-forming metastases from the initial tumor or independent primary growths, but found themselves baffled by the data.
In each mouse, these tumors typically shared numerous mutations, suggesting that they originated from a common progenitor. However, the mutational profiles of the metastatic growths strongly indicated that these secondary tumors emerged shortly after initial onset of melanoma, and were acquiring additional mutations along essentially the same timeline as the primary tumor. “We were unable to interpret our initial profiling results until we discovered—months later—that early tumor cell dissemination was the explanation,” Abastado says.
The SIgN-led team revealed the spread of cancer cells to tissues throughout the body in mice as young as three weeks of age. However, these early ‘colonies’ of disseminated tumor cells (Fig. 1) did not display the aggressive, invasive characteristics of true metastatic growths, suggesting that their progression was somehow being kept in check. Subsequent experiments demonstrated that this inhibition was largely due to the action of CD8+ T cells, and depletion of these immune cells from tumor-bearing RET.AAD mice led to rapidly accelerated tumor growth in the lung and reproductive tract.
Abastado suggests that a better understanding of this CD8+-imposed tumor dormancy may enable smarter therapeutic strategies. “We were very impressed by the fact that cancer is already a systemic disease at diagnosis, but can be controlled by the immune system for prolonged periods of time—and possibly the entire life,” he says. “This observation brings hope to those trying to mobilize the immune system against cancer.”
The A*STAR-affiliated researchers mentioned in this highlight are from the Singapore Immunology Network.



