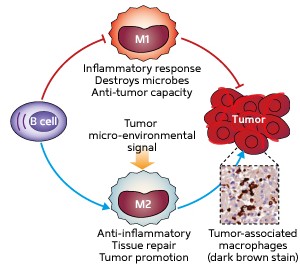
Fig. 1: Schematic diagram showing the two types of macrophages that can either defend the host from invading microbes and prevent tumor growth (M1) or promote tissue repair and tumor growth (M2). Their interaction with B cells is also shown.
© 2010 S. K Biswas
The immune system comprises two distinct branches that defend against infection in different ways. Cells of the innate immune system such as macrophages recognize microbes and destroy them. They also activate cells of the adaptive immune system such as T cells and B cells to provide longer-lasting defense.
The adaptive immune system also regulates the innate immune system, but in less clearly defined ways. Subhra K Biswas of the A*STAR Singapore Immunology Network (SIgN) and co-workers from the SIgN and the A*STAR Bioprocessing Technology Institute have now provided the first evidence that B cells play an essential role in regulating the physiological properties of macrophages.
Macrophages are broadly divided into two types. M1 macrophages are induced by microbes and chemicals released during inflammation, and have potent anti-microbial and anti-tumor properties; M2 macrophages are induced by exposure to anti-inflammatory molecules, and are involved in tissue repair but also promote tumor growth (Fig. 1).
Biswas and his co-workers isolated macrophages from normal mice and mutant animals lacking B cells, and stimulated them with bacterial lipopolysaccharides (LPSs) to induce an inflammatory response. Compared to normal animals, cells from the mutants expressed high levels of pro-inflammatory genes but low levels of the anti-inflammatory gene Il10, indicating that the absence of B cells produces an enhanced inflammatory response from macrophages.
The researchers then cultured macrophages with two different types of B cells (types B1 and B2) and studied their LPS response. They found that macrophages co-cultured with B1 cells and treated with LPS expressed low levels of the inflammatory genes and higher levels of Il10, compared with macrophages cultured with B2 cells or alone.
Further experiments revealed that B1 cells also induce the M2 type of macrophage in live animals by synthesizing and secreting IL-10 — which then induces its own expression, and that of other M2-phenotype specific genes — in macrophages.
Finally, Biswas and his colleagues investigated the effect of B1 cells on macrophages found in tumors by injecting mice with cancerous cells and then treating them with either B1 or B2 cells. In the animals injected with B1 cells, they observed increased expression of M2 phenotype genes and slightly increased tumor growth. Thus, B1 cells induced these macrophages to adopt a tumor-promoting M2 phenotype.
“Targeting macrophages to control cancer progression is an attractive strategy but very little is known about their role in human cancers,” says Biswas. “We are now investigating this, with the aim of identifying the molecular mechanisms that trigger macrophages into tumor-promoting mode.”
The A*STAR-affiliated researchers contributing to this research are from the Singapore Immunology Network and the Bioprocessing Technology Institute.



